Purpura fulminans is a rapidly progressive syndrome of small-vessel thrombosis and hemorrhagic necrosis of the skin accompanied by disseminated intravascular coagulation. We describe a case of Streptococcus pneumoniae septicemia in an asplenic 5-year-old boy on oral tacrolimus, with a past medical history of multivisceral organ transplantation and subsequent development of purpura fulminans on his chest and distal extremities. The acute infectious form of purpura fulminans is usually caused by gram-negative bacteria. Cases secondary to gram-positive encapsulated bacteria usually occur when individuals are immuno-suppressed or have anatomic or functional asplenia. Our patient had both, which likely increased his susceptibility, and he responded well to antimicrobial therapy in addition to prophylactic coverage in the setting of his immunosuppression. We review the literature for similar cases due to S. pneumoniae in the pediatric population and discuss the etiology and treatment of purpura fulminans.
La púrpura fulminante es un síndrome rápidamente progresivo de trombosis de pequeños vasos y necrosis hemorrágica de la piel que se acompaña de coagulación intravascular diseminada. Describimos un caso de septicemia por Streptococcus pneumoniae en un niño de 5 años de edad tratado con tacrolimus oral, con una historia médica previa de trasplante de múltiples vísceras y sin bazo, y el desarrollo subsiguiente de púrpura fulminante en su pecho y la parte distal de sus extremidades. La forma aguda infecciosa de púrpura fulminante es debida habitualmente a bacterias gramnegativas. Los casos secundarios a bacterias grampositivas encapsuladas ocurren por lo general cuando los individuos están inmunosuprimidos o presentan asplenia funcional o anatómica. Nuestro paciente presentaba ambas condiciones, lo cual con seguridad aumentó su susceptibilidad, y respondió bien a la terapia antimicrobiana además de a la cobertura profiláctica en el contexto de su inmunosupresión. Revisamos la literatura buscando casos similares debidos a Streptococcus pneumoniae en la población pediátrica y discutimos la etiología y el tratamiento de la púrpura fulminante.
A 5-year-old Hispanic boy with a past medical history of premature birth at 24 weeks gestation and multivisceral organ transplantation (MVTx) at 2 years of age due to a hepatoblastoma with extensive abdominal compromise was admitted at the ICU with a fever of 103.4° F (39.7°C). He complained of pain in his neck and right leg. He denied the onset of dysuria, cough, or ear pain. The MVTx included the stomach, pancreas, liver, and small and large bowels. He was on oral tacrolimus 1.0mg twice daily to prevent rejection, which was held on admission, and ganciclovir prophylaxis to prevent cytomegalovirus infection secondary to immuno-suppression. The transplant did not include a spleen and consequently he received a pneumococcal conjugate vaccine and was on penicillin prophylaxis due to increased risk of infection. He also had a right lower quadrant ileostomy with normal output and denied any gastrointestinal changes.
On initial examination he was in moderate distress, vasodilated, and hypotensive. After a sepsis workup, he was started with broad spectrum of empiric antibiotics including vancomycin, piperacillin, tazobactam, and ceftriaxone. Venous blood gases revealed a severe mixed acidosis and he was started on fluid resuscitation, pressor support, shock doses of hydrocortisone, and subsequently intubated. Lung edema worsened secondary to extracellular fluid leakage and worsening acute respiratory distress syndrome (ARDS) so transplant surgery placed bilateral pleural pigtail drains to improve ventilation. Hypocalcemia was addressed with continuous calcium infusion and renal failure secondary to sepsis was managed with continuous veno venous hemodiafiltration (CVVHDF).
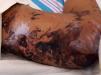
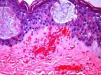
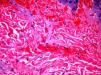
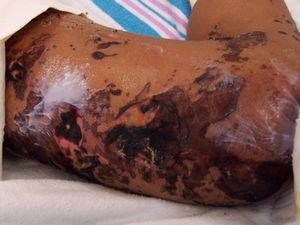
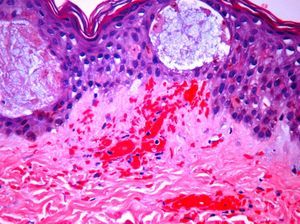
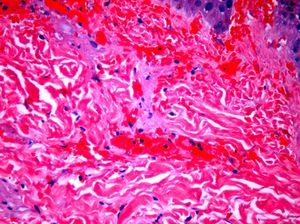
The day after admission, ecchymoses and petechiae with consolidated irregular areas of blue-black hemorrhagic necrosis and a surrounding erythematous border were noted on his chest and distal extremities (Fig. 1). He had cyanotic nailbeds with delayed perfusion. We did not have baseline quantitative or functional coagulation assays and levels of protein C, protein S, and antithrombin III were not measured. The rash progressed to numerous tense bullae, which were drained without debridement of the epidermis, and topical mupirocin was applied with Telfa dressing. The drained fluid was sent for culture and had no microbial growth. The dermatology service was consulted and a skin biopsy from the right arm revealed intraepidermal vesicles with focal epidermal necrosis, dermal hemorrhage, and multiple thrombosed vessels in the superficial and reticular dermis without evidence of vasculitis (Figs. 2 and 3).
Blood cultures came back as predominantly gram positive cocci in chains consistent with Streptococcus pneumoniae. The antimicrobial regimen was adjusted to linezolid, vancomycin, meropenem, abelcet, and gancyclovir for active coverage of S. pneumoniae septicemia and prophylactic coverage of other flora. He remained afebrile with a resolving leukocytosis and negative blood cultures and the affected areas continued to resolve with scattered hemorrhagic vesicles and crusts. Heparin or clotting factors were not administered because the patient was improving clinically with the adjusted antibiotic regimen by the time the skin biopsy results were obtained. He was weaned to lower settings on the ventilator, his heart rate and blood pressure stabilized, and tacrolimus was restarted due to subtherapeutic levels. Both pleural pigtail drains were removed without complications and CVVHDF was discontinued. He continued to have intermittent hemodialysis and was eventually extubated without complications.
DiscussionPurpura fulminans, also known as purpura gangrenosa, was first described by Guelliot in 1884 and is a rapidly progressive syndrome of intravascular thrombosis and hemorrhagic infarction of the skin. It often occurs in infants and small children and is accompanied by vascular collapse, fever and DIC. The disease is further classified into three forms: neonatal purpura fulminans, idiopathic purpura fulminans, and acute infectious purpura fulminans.1 We will focus on the third form, acute infectious purpura fulminans, which our pediatric patient was afflicted with.
Acute infectious purpura fulminans (AIPF) is the most common form and usually occurs in the setting of severe sepsis. It is characterized by large purpura, fever, hypotension, and DIC, although all four features may not be present in every patient. The most common infectious culprits are meningococcus and varicella, followed by gram-negative bacilli, staphylococci, Rickettsia, measles and streptococci, as described in our case.1 Including our case, from 1938 to 2011, there have been 19 documented pediatric cases of purpura fulminans in the setting of S. pneumoniae septicemia (Table 1). Anatomic or functional asplenia was present in 9 out of 19 patients (47%) and for those cases with documented outcomes, 11 out of 18 patients survived (61%). There was no correlation between the absence or presence of a spleen and survival rates – 5 of 9 asplenic patients survived (56%) and 6 of 9 splenic patients survived (67%).
Pediatric purpura fulminans cases due to Streptococcus pneumoniae.
| Source | Age | History and clinical presentation | Treatment | Outcome |
| Uhr, 19384 | 9/M | Sepsis | Sulfanilamide | Survived |
| Adner et al., 19703 | 5/M | Post-splenectomy for ITP; meningitis and sepsis | Heparin, penicillin G | Survived |
| Coonrod and Leach, 19765 | 16/M | Post-splenectomy for hypersplenism; sepsis | Died before treatment initiated | Died |
| Lanzkowsky et al., 19766 | Child | Post-splenectomy for Hodgkin's disease; sepsis | Died | |
| Cohen et al., 19907 | 2/F | Asplenic; sepsis | Died | |
| 8 month/M | Asplenic; sepsis | Survived | ||
| Ryan et al., 19938 | 1/F | Sepsis with Waterhouse–Friderichsen syndrome | Died | |
| Centers for Disease Control and Prevention, 19959 | 3 infants/children (<5 y) | Sepsis | Two died | |
| Cnota et al., 199910 | Child | Meningitis | Unknown | |
| Galanakis et al., 199911 | 6 month/F | Multiple organ failure, sepsis | FFP, aspirin, penicillin, ceftriaxone, dexamethasone | Survived |
| Pancharoen et al., 200212 | 3/F | Congenital asplenia; sepsis | Ceftriaxone | Survived |
| Noguera et al., 20042 | 13 month/M | Sepsis | FFP, cefotaxime, vancomycin | Survived |
| Lokeshwar et al., 200613 | 5 month/F | Sepsis | Penicillin G, APC | Survived |
| Meiners et al., 200614 | 11 month/F | Polysplenia; sepsis | Survived | |
| Bertran et al., 200915 | 3/F | Congenital asplenia, recurrent pneumonias; sepsis with Waterhouse–Friderichsen syndrome, multiple organ failure | PRBCs, FFP, cryoprecipitate, platelets, corticosteroids, cefotaxime | Died |
| Intan et al., 20091 | 7 month/M | Sepsis | PRBCs, FFP, penicillin, cefotraxime, vancomycin | Survived |
| Konda et al., 2011 (current case) | 5/M | MVTx without a spleen; ARDS, acute renal failure, sepsis | Linezolid, vancomycin, meropenem | Survived |
ARDS, acute respiratory distress syndrome; PRBCs, packed red blood cells; APC, activated protein C; FFP, fresh frozen plasma; MVT, multivisceral transplantation.
Gram-negative bacteria are the primary offenders in AIPF due to their ability to harbor endotoxins, such as lipopolysaccharide, in the outer membrane of their cell wall. These endotoxins are potent stimulators of the inflammatory cytokines interleukin (IL)-12, interferon-γ, tumor necrosis factor-α (TNF-α), and IL-1, which consume antithrombin III and proteins C and S. This alters the balance of anticoagulant and procoagulant activity, and ultimately results in a clinical picture of septic shock and DIC. The culprit in our case was a gram-positive encapsulated bacterium, S. pneumoniae, which is rare and often occurs in individuals with immuno-suppression or anatomic or functional asplenia – our patient was afflicted with both. Instead of producing endotoxin, S. pneumoniae releases a high-molecular-weight, glycan-teichoic acid fragment released by hydrolysis of the muramic acid-alanine bonds in the bacterial cell wall. This glycan-teichoic acid fragment is pro-inflammatory and propagates the production of TNF-α, IL-1, prostaglandins, and activation of the complement cascade. In return, vascular endothelial cells express increased cell surface leukocyte adhesion molecules, decreased surface co-factors for thrombin activation of protein C, decreased tissue-type plasminogen activator, and increased type 1 plasminogen inhibitor. This cascade ultimately results in DIC and the production of purpura.2
Treatment recommendations for prothrombotic disorders underlying purpura fulminans are still evolving. Management includes supportive therapy, replacement of blood products and clotting factors as appropriate, correction of acid–base and electrolyte abnormalities, and early use of oxygen and mechanical ventilation. Heparin is usually administered as a standard initial therapy to inhibit thrombus formation and consumption of coagulation factors. To avoid further thrombosis, some clinicians favor administering heparin prior to replacement of clotting factors. Protein C has both anti-inflammatory and anticoagulant properties and low levels can be repleted with plasma-derived protein C zymogen concentrates or drotecogin alpha (activated). Fresh frozen plasma (FFP) is also a source of protein C and protein S, but frequent administration is required to maintain adequate plasma levels. Antithrombin III replacement has also been shown to normalize levels and reverse DIC. Patients who need long-term anticoagulation are transitioned to warfarin therapy with overlapping protein C substitution to prevent warfarin-induced skin necrosis.
Documentation of treatment was obtained for 9 out of 19 patients (Table 1). Treatment modalities included antibiotics, heparin, fresh frozen plasma, cryoprecipitate, and activated protein C. Even though heparin is generally recommended as a standard initial therapy, it was administered to only one patient – in a case from 1970.3 Similarly, only one patient received activated protein C, three received FFP, and one received FFP and cryoprecipitate. Interestingly, 5 of 6 patients survived (83%) after receiving heparin or clotting factors with antibiotics and 3 of 3 patients survived (100%) after receiving antibiotics alone, including our patient. Larger sample sizes are warranted to determine the impact of each of these therapies on survival outcomes of pediatric patients with purpura fulminans.
Additional therapies have been shown to slow the progression of the disease including, recombinant tissue plasminogen activator, prostacyclin, topical nitroglycerin, IV dextran, plasmapheresis, and regional anesthesia. Epsilon aminocaproic acid, vitamin K, ketanserin, hyperbaric oxygen, leech saliva (hirudin), and glucocorticoids have also been utilized with limited anecdotal benefit.
Necrotic tissue should be promptly excised with subsequent wound closure. Further surgical procedures such as escharotomies, fasciotomies, or major amputations might be required once the patient has been stabilized. When amputation is necessary, stump wounds may heal by secondary intention from wound debridement, split-thickness skin grafting, tissue and muscle flaps, plantar skin free transfer, skin expansion, artificial skin, hyperbaric oxygen therapy, and in difficult cases, tissue-engineered skin. In our opinion, early diagnosis and aggressive antibiotic treatment were key factors in the successful outcome of our patient.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Konda S, Zell D, Milikowski C, Alonso-Llamazares J. Púrpura fulminante en relación con septicemia por Streptococcus pneumoniae en un paciente pediátrico asplénico. Actas Dermosifiliogr. 2011. http://dx.doi.org/10.1016/j.ad.2012.05.009.