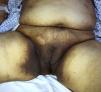
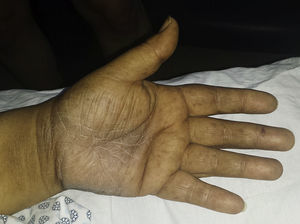
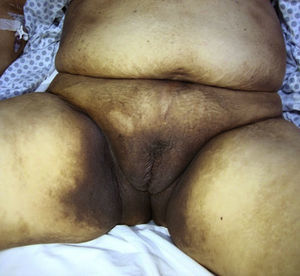
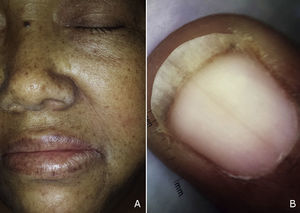
The patient was a 59-year-old woman with a history of type 2 diabetes mellitus, hypertension, and gout who was receiving treatment with metformin, valsartan, enalapril, and allopurinol. She consulted a dermatologist 1 month after developing hyperpigmented macules. While the lesions appeared initially on the palms of the hands and soles of the feet, the patient subsequently developed widespread cutaneous involvement, predominantly in intertriginous areas (Figs. 1 and 2). Of particular note were hyperpigmented macules on the lips and longitudinal melanonychia on the fingernails (Fig. 3). The differential diagnosis was lichen planus pigmentosus-inversus or an adverse drug reaction of the liquenoide type. Two skin biopsies showed a superficial spongiotic and psoriasiform perivascular dermatitis with eosinophils, consistent with an adverse drug reaction. On follow-up at 6 months, the patient presented mucocutaneous jaundice, asthenia, and dyspnea at rest. A series of tests were ordered and the results revealed severe megaloblastic anemia (Table 1). In the etiologic study, high titers of antibodies to parietal cells and intrinsic factor were found, and upper digestive tract endoscopy showed chronic atrophic inflammation of the gastric mucosa. The results of the following tests were normal: iron, folate, cortisol, thyroid-stimulating hormone, free thyroxine, rapid plasma reagin, and serological assays for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus. Treatment with intravenous vitamin B12 was started. The patients progressed satisfactorily, with partial regression of the skin lesions and complete remission of hematologic abnormalities after 5 months of follow-up (Table 1).
Summary of Laboratory Test Results on Diagnosis and 5 Months Posttreatment.
| Parameter | Pretreatment | Aftercare | Normal Range |
|---|---|---|---|
| Erythrocyte count (x106/mm3) | 1.52 | 5.5 | 4.0–5.2 |
| Hemoglobin (g/dL) | 6.0 | 13.0 | 12–16 |
| Hematocrit (%) | 17.4 | 41.5 | 36–46 |
| Mean corpuscular volume (fL) | 114.5 | 75.5 | 80–100 |
| Reticulocytes (%) | 3.06 | 1.12 | 0.5–1.5 |
| Serum B12 levels (pg/mL) | 105 | 559 | 200–900 |
| Blood film | Anisocytosis, polychromasia, macrocytosis, dacrocyte, hypersegmented neutrophils | Hypochromia, polychromasia, microcytosis |
Vitamin B12 plays an important role in the synthesis of DNA and RNA, acting as an enzyme cofactor.1 Onset of vitamin B12 deficiency is usually subacute, starting when the body's reserves are exhausted. The deficiency can be caused by a range of nutritional problems, including a lack of intrinsic factor, achlorhydria, ileal disease, malnutrition, and malabsorption syndromes.2 Diagnosis and treatment are of great clinical importance, mainly because of the progressive hematologic and neurologic involvement.
The following cutaneous manifestations have been described: generalized hyperpigmentation, glossitis, nail abnormalities, and premature graying. Hyperpigmentation is often more pronounced on the limbs, primarily on the dorsum of the hands and feet, in flexural areas, and occasionally on the nails, tongue, and oral mucous membranes.3 Since hyperpigmentation is a nonspecific symptom, a broad range of differential diagnoses should be considered in such cases: diabetes mellitus, Addison disease, Cushing syndrome, post-inflammatory lesions, amyloidosis, melanosis of the skin, heavy metal deposition, thyroid disease, tumors, drug reactions, and porphyria cutanea tarda, among others.4
The pathophysiology of hyperpigmentation is still a matter of debate. The first hypothesis was proposed by Gilliam and Cox,5 who reported that patients with vitamin B12 deficiency had decreased levels of reduced glutathione (GSH). It is known that GSH inhibits tyrosinase activity and consequently melanogenesis. Low levels of GSH permit increased tyrosinase activity, and thereby favor an increase in melanogenesis. Griepp6 proposed a hypothesis involving biopterin, a substance essential to the hydroxylation of phenylalanine. Given the role of phenylalanine in the synthesis of melanin, high levels of this amino acid could explain hyperpigmentation. Marks3 hypothesized that a change in the location and distribution of melanin might be involved, observing that megaloblastic anemia is associated with a defect in the transport of melanin and its incorporation into keratinocytes.
Accurate recognition of the cutaneous manifestations and eventual systemic involvement is essential in establishing a diagnosis. Blood tests may reveal macrocytosis, immature nuclei, and hypersegmented granulocytes. There may be an increase in levels of serum bilirubin and lactate dehydrogenase, as occurred in the present case. The sensitivity of low serum vitamin B12 concentrations (< 200(g/mL) ranges from 65% to 95%,1 making it necessary to complement this finding with other tests having greater sensitivity. Levels of methylmalonic acid higher than 400nmol/L and homocysteine above 21μmol/L have a sensitivity of 98% and 96%, respectively.1 Once the diagnosis is confirmed, the cause of the deficiency should be investigated. In severe cases, it should be remembered that the most common cause of vitamin B12 deficiency is autoimmune gastritis.7
In our patient, further tests and explorations revealed high titers of antibodies against parietal cells and intrinsic factor and chronic atrophic inflammation of the gastric mucosa. These findings supported a diagnosis of autoimmune gastritis. However, it should be noted that the patient was receiving treatment with metformin, a drug that has been associated with decreased serum levels of vitamin B12. A recent meta-analysis found treatment with metformin to be significantly associated with an increased incidence of vitamin B12 deficiency and reduced serum levels.8 It is therefore possible that the etiology in this case may have been twofold.
Histology may reveal epidermal thinning, vacuolar changes and nuclear elongation of keratinocytes, increases in the number of melanocytes in basal layers, and the presence of numerous melanophages in the papillary dermis.3,9 Electron microscopy will show intracytoplasmic desmosomes, numerous aggregated bundles of tonofilaments in the cytoplasm of keratinocytes, and highly condensed keratohyalin granules.10 In the present case, the results of the 2 histological studies carried out were not consistent with the findings described above and were, rather, indicative of an adverse drug reaction. The possible complementary role in the etiology of 1 of this patient's medications could explain these findings, and the histological changes associated with the drug reaction may have masked those more often found in cases of vitamin B12 deficiency.
Recommended treatment is based on the administration of vitamin B12 either orally, intravenously, or intramuscularly; a number of management protocols exist. In a randomized clinical trial comparing oral and parenteral therapy, both groups showed similar reductions in mean corpuscular volume and increases in hematocrit at 4 months.11
The present case highlights the causal relationship between vitamin B12 deficiency and generalized skin hyperpigmentation, an association that presents a wide variety of dermatological manifestations. Clinical suspicion plays a fundamental role in the diagnostic process, and is higher in patients at higher risk, including vegetarians, malnourished patients, older people, and patients who have malabsorption syndromes or have undergone gastrectomy or bariatric surgery.
Please cite this article as: Vera-Kellet C, Andino-Navarrete R, Navajas-Galimany L. Déficit de vitamina B12 y sus diversas manifestaciones dermatológicas. Actas Dermosifiliogr. 2015;106:762–764.