Topical agents are the first-line treatment for mild and moderate psoriasis, but factors such as frequency of administration, organoleptic properties, and the limited short term results can reduce treatment adherence and effectiveness.
Innovations in topical treatments are linked not only to the discovery of new molecules, but also to the reformulation of existing active ingredients based on improvements to administration, organoleptic properties, bioavailability, and ease of use. Calcipotriol and betamethasone dipropionate aerosol foam is a new formulation in which the active ingredients are dissolved in a mixture of volatile propellants that evaporate quickly, leaving a supersaturated solution of calcipotriol and betamethasone dipropionate that enhances penetration into the epidermis.
In this article, we take a look at the new calcipotriol and betamethasone dipropionate aerosol formulation and briefly review the main evidence supporting the use of topical treatments for psoriasis.
Los agentes tópicos representan la primera línea de tratamiento para la psoriasis leve y moderada. Sin embargo, factores como la frecuencia de administración, las características organolépticas o los resultados limitados a corto plazo pueden disminuir la adherencia al tratamiento y su efectividad.
Las innovaciones en los tratamientos tópicos se basan en descubrir nuevas moléculas, pero también en la reformulación de principios activos ya conocidos, mejorando su administración, características organolépticas, biodisponibilidad y comodidad de uso. La espuma en aerosol de calcipotriol y dipropionato de betametasona es una nueva formulación donde los principios activos están disueltos en una mezcla de propelentes volátiles que se evaporan rápidamente dejando una solución sobresaturada de calcipotriol y dipropionato de betametasona, que aumenta la penetración de los fármacos en la epidermis.
En este artículo se hace un breve repaso de las principales evidencias sobre los tratamientos tópicos disponibles para la psoriasis y de la nueva formulación en espuma de calcipotriol y dipropionato de betametasona.
Psoriasis is a chronic, immunologically mediated inflammatory disorder with a genetic basis. The estimated prevalence in Spain is 2.3% (mild disease in 55.6% of patients, moderate in 37.6%, and severe in 6.6%).1–3 Whereas mild psoriasis can be treated topically, moderate to severe disease usually requires adjuvant therapy with systemic drugs and/or phototherapy).4 Low systemic absorption is the advantage of topical treatment, but its disadvantages (frequent applications, inconvenience, unpleasant organoleptic properties, and limited short-term efficacy) can lead to poor adherence and undermine effectiveness.5
Currently available topical therapies include a wide range of formulations, drug combinations, and levels of potency (Table 1).6,7 The choice of one over another should be made according to patient needs and characteristics.8
Summary of the Main Properties of Topical Psoriasis Treatments
| Mechanism of Action | Efficacy vs Placeboa | Efficacy vs Other Options | Recommendations | Drawbacks | |
|---|---|---|---|---|---|
| Highly potent topical corticosteroids | Anti-inflammatory and immunomodulatory: modulate genetic transcription of anti-inflammatory molecules and ↓proinflammatory cytokines Nongenic effects on dermal fibroblasts and vessels | Meta-analysis: 10 studies (n=1264) Effect size (95% CI): −1.56 IGA score: 1.81 | >Potent corticosteroids <Corticosteroid+vitamin D analog >Vitamin D analogs | Not for use on skin folds or face. Body+scalp psoriasis Dose: 1 application/d Short term: 4 wk Intermittent long-term regimen | Rebound effect Tachyphylaxis Skin atrophy Striae Telangiectasia Possible systemic effects |
| Potent topical corticosteroids | Meta-analysis: 13 studies (n=2216) Effect size (95% CI): −0.89 IGA score: 1.02 | <Highly potent corticosteroids <Corticosteroid+vitamin D analogs <>Vitamin D analogs >Vitamin D analogs on scalp | |||
| Vitamin D analogs | Effect on genetic transcription, keratinocytes: ↓cell hyperproliferation, ↑cell differentiation, ↓production of proinflammatory cytokines | Meta-analysis: 30 studies (n=4986) Effect size (95% CI): −0.90 IGA score: 1.03 | <Highly potent corticosteroids <Corticosteroid+vitamin D analogs <Potent or highly potent corticosteroids on scalp <>Potent corticosteroids <>Dithranol | Body psoriasis Dose: 1 or 2 times/d Duration: no limit Not in children under 6 y | Skin irritation: pain and itching at site of application |
| Combined corticosteroid+vitamin D analog (Cal/BD) | Complementary anti-inflammatory and immunomodulatory effects: ↑rapid onset, ↑enduring posttreatment effect, ↓rebound effect | Meta-analysis: 5 studies (n=2058) Effect size (95% CI): −1.44 IGA score: 2.18 (2 times/d), 1.39 (1 time/d) | >Highly potent corticosteroids <Potent or highly potent corticosteroids on scalp >Potent corticosteroids >Vitamin D analogs >Dithranol | Body+scalp psoriasis Dose: 1 or 2 times/d Fixed combination or separately Duration: no limit | AE (fewer than if separate): pain and itching on site of application |
| Dithranol | ↓hyperproliferation of keratinocytes, ↓granulocytic function Immunosuppressant | Meta-analysis: 3 studies (n=47) Effect size (95% CI): −1.06 IGA score: 1.22 | <Highly potent corticosteroids <Potent corticosteroids <>Vitamin D analogs | Best in combined therapy (corticosteroids, vitamin D analogs, phototherapy) Dose: 1–2 times/d For use in specialist clinics | Local irritation Staining (clothing, furnishings) |
| Tazarotene | Regulates proliferation and differentiation of keratinocytes | Meta-analysis: 1 study (n=318) Effect size (95% CI): −0.86 TSS: 3.8 vs 5.3 | <Highly potent corticosteroids <Potent corticosteroids <Vitamin D analogs <Dithranol | Gel formulation Long-lasting effect after treatment Dose 1 time/day Combined with corticosteroids Indication: soles, palms, nails | Low efficacy Local irritation |
Abbreviations: Cal/BD, calcipotriol plus betamethasone dipropionate; IGA, investigator's global assessment of overall improvement; TSS, total severity score.
One Cochrane review concluded that combinations of vitamin D and corticosteroids are generally more effective than monotherapies using the same active ingredients applied separately.6 The probable rate of response to combined treatment (70.9%) approximates that of highly potent corticosteroids (67.9%) when both ingredients are applied once daily.7
The calcipotriol and betamethasone dipropionate (Cal/BP) combination achieved a mean reduction in the Psoriasis Area and Severity Index (PASI) of 65% to 74% at 4 weeks in several studies according to another review.8 Irritation and itching were the most common adverse effects. This combination has also been reported to reduce epidermal and dermal thinning less than BD alone in long-term use.9
Current guidelines and consensus statements recommend topical corticosteroids and vitamin D analogs alone or in combination as the best choices for treating psoriasis.6,7,10–12
Innovations in Approaches to Topical Treatment of Psoriasis: The Contributions of Cal/BD FoamFailure to adhere to treatment affects 39% to 73% of patients prescribed topical therapy for psoriasis.13,14 Many studies in recent years have therefore focused on improving adherence to familiar topical therapies15 by increasing the bioavailability of active ingredients16,17 to enhance efficacy, hasten onset of action and improve the safety profile, as well as to lower doses and make the products easier to apply.5
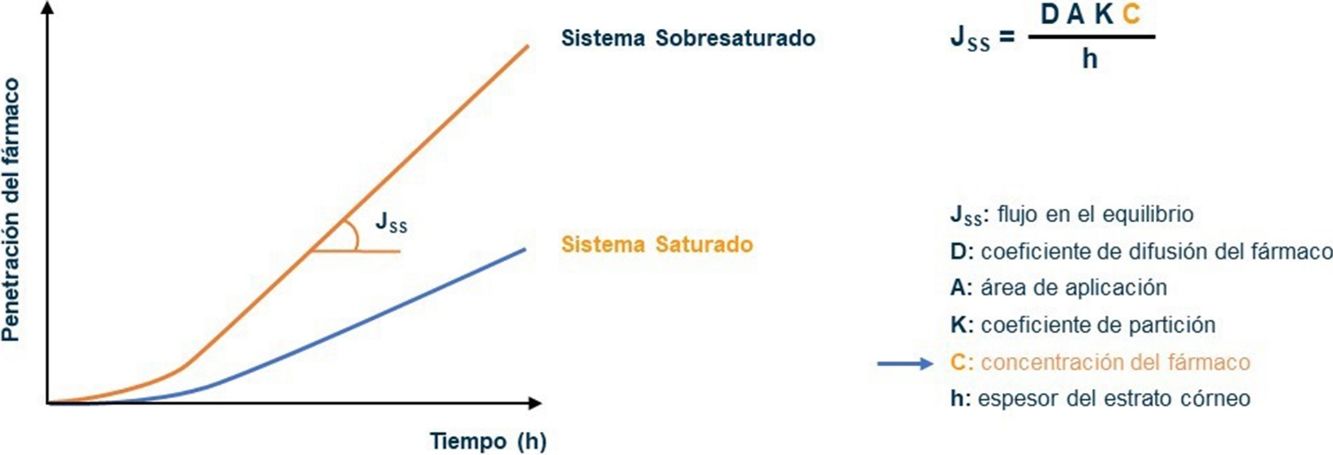
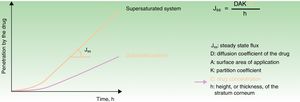
The bioavailability of active ingredients in a vehicle for topical application on the skin is determined by the ability of the product to penetrate the epidermis, among other factors.16–18 The Cal/BD aerosol foam contains 2 well known active ingredients combined with volatile propellants (butane and dimethyl ether). The propellants evaporate soon after the product is applied, leaving a thin layer of foam consisting of a supersaturated solution of Cal/BD. Because both active compounds are available at high concentrations, they penetrate the stratum corneum more rapidly, in accordance with Fick's law (Fig. 1).17–23 The process involves dissolving the drugs in volatile cosolvents to allow the active ingredients to remain in the vehicle at levels that exceed the maximum solubility without precipitating. Two studies—one in vitro and the other a phase I trial—have demonstrated, respectively, that vasoconstrictor potency and rate of penetration are superior with foam application compared to the same combination of ingredients in creams or ointments.20,23
Fick's law model of the passage of substances through membranes, showing the diffusion–concentration relationship. The law postulates that substances flow from an area of high concentration to areas of low concentration to a degree that is proportional to the concentration gradient. This is to say, the higher the concentration, the higher the diffusion of the drug through the membrane, and by analogy, of the active ingredient through the epidermis.
A first exploratory study showed that a Cal/BD foam reduced the total clinical score (a composite of erythema, scaling, and thickness) significantly more than a Cal/BD ointment (P=.038), a foam containing BD alone (P=.005), or the foam vehicle alone (P<.001).22 Ultrasound measurements confirmed greater reduction of the thickening and inflammation associated with psoriasis in that study.
Further clinical trials have also demonstrated that the Cal/BD foam formulation performed better than the ingredients alone in foam, the Cal/BD ointment or gel, or the vehicle alone14,24–28 (Table 2).
Summary of the Main Evidence Base for the Development of the New Cal/BD Aerosol Foam Formulation
| Reference | Study Design | No. of Subjects | Treatments | Dose Regimen and Duration | Findings | |
|---|---|---|---|---|---|---|
| Pharmacokinetics and bioavailability | ||||||
| Penetration | Hollesen Basse et al. J Invest Dermatol.2014(ref.20) | In vitro, phase I, pig ear skin | Cal/BD foam vs Cal/BD ointment | Single application Controls at 2h, 6h, 21h | Greater penetration of foam vs ointment (P<.05) Stability: foam levels constant, 2–21h | |
| Vasoconstrictor potency | Queille-Roussel et al. J Eur Acad Dermatol Venereol.2016(ref.23) | Phase I, single-center, researcher-blinded, controlled, intraindividual comparisons | 35 healthy volunteers | Cal/BD foam vs Cal/BD ointment vs clobetasol cream vs fluocinolone ointment vs BD foam vs foam vehicle vs negative control | Single application at different locations, 6–32h postapplication | Vasoconstrictor potency: clobetasol cream>Cal/BD>fluocinolone ointment |
| Efficacy | ||||||
| Exploratory study | Queille-Roussel et al.Clin Drug Investig.2015 (ref.22) | Phase IIa, exploratory, single-center, researcher-blinded, controlled Intraindividual comparisons | 24 patients | Cal/BD foam vs Cal/BD ointment vs BD foam vs foam vehicle | 1 application/d, 4 wk | Total clinical score: Cal/BD foam: −6±1.27 vs Cal/BD ointment: −5.25 (P<.05) vs BD foam −4.96 (P<.05) vs foam vehicle −1.88 (P<.0001) |
| Vs single active components | Lebwohl et al. J Clin Aesthet Dermatol. 2016 (ref.14) | Phase II, multicenter, randomized, double-blind | 302 patients | Cal/BD foam vs Cal foam vs BD foam | 1 application/d, 4 wk | Success rate/mPASI75 (4 wk): Cal/BD foam, 45%/49% vs Cal foam, 14.9%/18% (P<.001) vs BD foam, 30.7%/34% (P<.05) |
| Vs Cal/BD ointment | Koo et al. J Dermatolog Treat. 2016 (ref.25) | Phase II, multicenter, randomized, researcher-blinded | 376 patients | Cal/BD foam vs Cal/BD ointment vs foam vehicle vs ointment vehicle | 1 application/d, 4 wk | Success rate/↓mPASI (4 wk): Cal/BD foam, 54.6%/↓74.2% vs Cal/BD ointment 43%/↓63.2% (P<.05) |
| Vs vehicle PSO-FAST study | Leonardi et al. J Drugs Dermatol. 2015 (ref.26); J Drugs Dermatol. 2016 (ref.29) | Phase III, multicenter, randomized, double-blind | 426 patients | Cal/BD foam vs foam vehicle | 1 application/d, 4 wk | Results (wk 4) Success: 53.3% vs 4.8% (P<.001) mPASI: 2 vs 5.5 (P<.001) mPAS75: 52.9% vs 8.2% (P<.001) ↓70% pruritus: 83.5% vs 40.6% (P<.001) DLQI (0/1): 48.1% vs 21.2% (P<.001) |
| Vs Cal/BD gel, PSO-ABLE study | Paul et al. J Eur Acad Dermatol Venereol.2017 (ref.27) | Phase III, multicenter, randomized, researcher-blinded | 463 patients | Cal/BD foam vs Cal/BD gel vs foam vehicle vs gel vehicle | 1 application/d, 12 wk | Results (4 wk vs 8 wk) Success: 38% vs 22% (P<.001) mPASI75: 52% vs 35% (P<.001) mPASI90: 22% vs 11% (P<.001) Success (12 wk): 44% vs 34% |
| Paul et al. Am J Clin Dermatol. 2017 (ref.30) | PSO-ABLE subgroup analysis | 159 patients, moderate to severe psoriasis | Cal/BD foam (n=77) vs Cal/BD (n=82) | 1 application/d, 12 wk | Success (4 wk): 32% vs 19% Success (4 wk): 36% vs 17% (P<.05) Success (12 wk): 38% vs 32% mPASI75 (4 wk 4): 40% vs 17% (P=.001) mPASI75 (8 wk): 53% vs 22% (P<.001) mPASI75 (12 wk): 57% vs 35% (P<.01) | |
| Pooled data analysis | Stein Gold et al. J Drugs Dermatol. 2016 (ref.28)a | Pooled-data analysis of Koo et al (ref.25) + Lebwohl et al (ref.14)+ Leonardi et al (ref.26) | 1104 patients | Cal/BD foam vs Cal foam vs BD foam vs Cal/BD ointment vs foam vehicle | 1 application/d, 4 wk | Success rate/↓ mPASI/PASI75 (4 wk): Cal/BD foam, 51%/↓72%/51% vs Cal/BD ointment, 43%/↓63%/41% vs BD foam, 31%/↓53%/34% vs Cal foam, 15%/↓43%/18% vs vehicle foam, 5%/↓32%/7% vs vehicle ointment, 8%/↓33%/10% |
| Safety | ||||||
| Tolerability data from efficacy trials | Menter et al. Skinmed 2017(ref.33)a | Pooled-data analysis of Koo et al (ref.25)+Lebwohl et al (ref.14)+ Leonardi et al (ref.26) | 1104 patients | Cal/BD foam vs Cal foam vs BD foam vs Cal/BD ointment vs foam vehicle | 1 application/d, 12 wk | AE: Cal/BD foam, 13.8% (NS vs others) ADR: Cal/BD foam 2.7% vs 3% Cal/BD ointment vs 6.1% Cal foam vs 7.1% BD foam Most common ADR to Cal/BD foam: pain (4/564) and pruritus (2/564) at application site |
| Potential for irritation and sensitization | Queille-Roussel et al. J Am Acad Dermatol. 2015 (ref.32) | Phase I, single center, randomized, controlled | 213 healthy volunteers | Cal/BD foam vs foam vehicle vs white petroleum jelly (negative control) | 5 applications/wk (semiocclusive patches) for 3 wk+2 wk rest+1 application | Low irritative potential Maximum score, 2 (erythema with mild to moderate edema in 3/213 volunteers) |
| MUSE (Maximum Use and Systemic Exposure) trial | Taraska et al. J Cutan Med Surg. 2016 (ref.34) | Phase II, multicenter, open-label, uncontrolled | 35 patients, moderate to severe psoriasis BSA: 15%–30% | Cal/BD foam | 1 application/d 4 wk 13.5–113g/wk | ACTH challenge test: normal Albumin-corrected calcium level : normal 24-h urinary calcium: normal Calcium– creatinine ratio in urine: normal |
Abbreviations: ACTH, adrenocorticotropic hormone; AE, adverse events; BSA, body surface area; mPASI, Psoriasis Area and Severity Index modified by ignoring psoriasis on the head; ADR, adverse drug reaction.
More patients (45%) using the Cal/BD foam had clear or nearly clear skin (physician's global assessment [PGA], 0/1) and had also improved at least 2 points in the PGA score after 4 weeks than patients using a Cal-only foam (14.9%, P<.001) or a BD-only foam (30.7%, P=.047) in a phase-II trial.21 The reduction in the modified PASI (mPASI) and the proportion of patients with mPASI75 improvement were also greater with the Cal/BD foam (P<.001) in another trial.14
A phase-II trial comparing the Cal/BD combination in foam or ointment found that more patients had a successful response (PGA, 0/1) to the foam (54.6%) than to the ointment (43%) (P<.05) or to a placebo ointment or foam.25 The formulations had similar safety profiles. The mPASI reduction was significantly greater with the foam formulation from the first week (−43.4% vs −30.5% for the ointment, P<.001) through the fourth week (−74.2% vs −63.2%, P<.01).
The phase III PSO-FAST trial26 confirmed the efficacy of the Cal/BD foam, reporting a success rate of 53.3% in the active-treatment arm (vs 4.8% in the vehicle arm, P<.001) and a final mPASI of 2 after 4 weeks (vs 5.5, P<.001). Rapid and significant reduction in pruritus was reported by 83.5% of patients in the intervention arm, and their quality of life also improved. The Dermatology Life Quality Index (DLQI) rose 7 points with treatment but only 4.4 points with the vehicle (P<.001), and 48.1% of the intervention patients reported little or no effect of the disease on their quality of life (DLQI, 0/1) after 4 weeks (vs 21.2% of vehicle patients, P<.001).29
The PSO-ABLE study found that Cal/BD foam application was more effective at 4weeks than the Cal/BD gel at 8weeks, in terms of both the therapeutic success rates (38% vs 22%, respectively) and the proportion of patients who reached mPASI75 (52% vs 35%) and mPASI90 (22.2 vs 10.7%) with respecto to baseline values (P<.001, all comparisons).27 The 12-week success rates were 44.1% for the Cal/BD foam and 34.3% for the gel. A patient preference survey showed that 83.7% preferred the Cal/BD foam to other therapies they had tried and that they perceived it to be more effective (88.3%), easier to apply (76.5%), and easier to tolerate (81.0%). A subsequent subanalysis of the dataset for patients with moderate to severe psoriasis (10% or more of body surface area involvement and/or PASI or DLQImore than10) showed that more patients improved (mPASI75) at 4, 8, and 12weeks on the Cal/BD foam than on the gel (57.1% vs 35.4%; P=.006 at week 12). The rate of therapeutic success, reflected by PGA 0/1, was higher for Cal/BD foam only at week 8. PGA 0/1 response rates were as follows: week 4, 32% vs 19%; week 8, 35.6% vs 16.9% (P<.05), and week 12, 38% vs 31.6%.30 Another subanalysis of tpatients with the most severe disease in the dataset found that at week 12 there were reductions in proinflammatory markers (interleukin 17A and macrophage-derived chemokine CCL22; P<.0001) and that levels of adiponectin, a marker with cardioprotective effects, increased (P=.03).31 Prospective placebo-controlled trials are needed to confirm these results.
Safety of Cal/BD FoamCal/BD foam has been shown to have low irritative and sensitizing potential.32 Phase II and III efficacy studies have demonstrated an acceptable safety profile and good tolerability in treatments lasting up to 12 weeks.14,25–27 A pooled analysis of data from 3 clinical trials enrolling a total of 1104 patients treated for 4 weeks showed that the most common adverse events among users of the Cal/BD foam were nasopharyngitis (n=6; 1.1%) and pain at the site of application (n=4; 0.7%).33 The rate of adverse drug reactions was 2.7%. The most common ones were pain (n=4; 0.7%) and puritis (n=2; 0.4%) at the site of application. The overall safety profile of the Cal/BD foam was similar to the profiles of the gel and ointment formulations. No local or systemic adverse effects after long-term use of the foam were detected in the PSO-ABLE study.27
Finally, the phase II Maximum Use and Systemic Exposure (MUSE) study assessed the function of the hypothalamic–pituitary–adrenal (HPA) axis and calcium homeostasis at maximum doses of the Cal/BD foam (13.5–113g/wk) for 4weeks.34 No clinically important effects on the HPA axis, albumin-corrected serum calcium level, or the 24-hour urinary calcium–creatinine ratio were detected.
DiscussionTopical treatments continue to be cornerstones of day-to-day management of psoriasis, particularly in mild cases or as a complement to systemic treatment. The main advantages of topical formulations are the reduced risk of toxicity and the better support for patient independence in disease management. One disadvantage is lower efficacy in comparison with systemic treatments—although no clinical trials comparing the two have been done. Another disadvantage is the possibility of lower adherence to therapy.
Developing new topical formulations that are more effective and convenient for psoriasis patients is a high-priority goal. One current direction of innovation is the reformulation of known drugs15 to enhance product efficacy by improving the bioavailability of active ingredients applied to the skin. Reformulation also targets improved organoleptic properties. Both these factors have an impact on patient preferences and increase adherence.
This review has evaluated information related to the clinical development of the Cal/BD combination in a new foam vehicle and aerosol applicator. Studies demonstrate that efficacy is adequate and superior to that of existing gel and ointment forms, encouraging acceptance during long-term use.
These improvements result from the creation of a supersaturated steady-state and entirely bioavailable solution of Cal/BD after the foam is applied to the skin. The formulation thus has a faster rate of penetration of the stratum corneum without compromising safety.
Additional clinical trials are under way. One is assessing the long-term effects of Cal/BD foam for maintenance therapy (NCT0289962) and another is evaluating efficacy in children (NCT02387853).35
Several topical treatments for psoriasis are presently under development.36 The anti-inflammatory agent cisaborole—a phosphodiesterase-4 inhibitor approved by the US Food and Drug Administration for treating atopic dermatitis—is in phase II studies for use in psoriasis (NCT01300052). Others are PH-10 (rose Bengal disodium) in a hydrogel (NCT01247818) and SNA-120 (pegcantratinib) and tofacitinib in ointment formulations (NCT03322137 and NCT01831466, respectively). Three formulations are in phase III studies: IDP-118, a combination of halobetasol propionate and tazarotene in lotion (NCT02462083); M518101 (NCT01908595); and MC2-01, a Cal/BP combination in cream (NCT03308799).
Conflicts of InterestThis paper was drafted with the help of BlueBliss, with funding from LEO Pharma.
Dr Puig has received consultancy and speaker's honoraria from LEO Pharma. His hospital has also received funding from LEO Pharma for research in clinical trials. Dr Carretero has served as a consultant or given talks for LEO Pharma and Gebro Pharma.
Lidia Rodriguez, Cluster Medical Advisor, Dermatology, for LEO Pharma, Southern Europe, helped with editing the manuscript.
Please cite this article as: Puig L, Carretero G. Actualización del tratamiento tópico en psoriasis: aportación de la combinación de calcipotriol y dipropionato de betametasona en espuma. Actas Dermosifiliogr. 2019;110:115–123.