The development of new information and communication technology has led to better, properly validated measurement tools. However, such tools are sometimes created in languages other than our own and in countries that are culturally distinct from those of populations we wish to study. This situation creates a need to adapt assessment tools before we can use them in new contexts.
An adapted instrument, rather than an entirely new one, is needed because the indiscriminate creation of questionnaires is unjustified. The development of a new tool takes longer, is more costly, and uses more nonmonetary resources as well; moreover, the result would not be useful for generating knowledge.1 Consider, for example, the case of a 2010 Cochrane Collaboration systematic review of vitiligo treatments that concluded that trial results could not be compared because the researchers had assessed repigmentation using 48 different scales.2
An appropriate process must be followed to ensure that a translated measurement tool uses language in the way it is understood in a cultural context that is different from the original setting yet does not lose its measurement properties.3
Herdman et al5 used an approach they described as universalist to achieve equivalence when adapting an instrument. Their approach contrasted with the previously followed one they called absolutist, which had focused on ensuring that little or no change in the original concepts and organization would be made when producing the adapted version. Language issues were the primary concern of the absolutist approach, which often led to problems with adaptation.5 The universalist approach recognizes that concepts can vary from one culture to another and can have a different scope of meanings, or might not even exist; even countries that share the same language might have diametrically opposed meanings attached to items or they might not recognize certain associated meanings for cultural or social reasons.6 Therefore, the universalist approach Herdman and colleagues proposed first selects elements related to a construct that are truly universal and then adapts only those that measure the same concept in both cultures.5 This focus ensures greater equivalence between different language versions of a measurement tool.
When Epstein et al4 reviewed guidelines for the cross-cultural adaptation of assessment tools, they identified 31 different approaches. The aim of the present article is to simplify the proposed processes currently on offer and suggest one that leads to a culturally adapted translation that can later be validated for use in the intended setting. We will not lengthen this article by discussing the validation of measurement tools at this time.
Our proposals are based on the following 3 sources:
- •
the principles of good practice in the translation and cultural adaptation of patient-reported outcome measures, as set out by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR),7
- •
the guidelines for the translation and adaptation of tests by the International Test Commission,8 and
- •
the guidelines for the cross-cultural adaptation of self-report measures published by the journal Spine.9
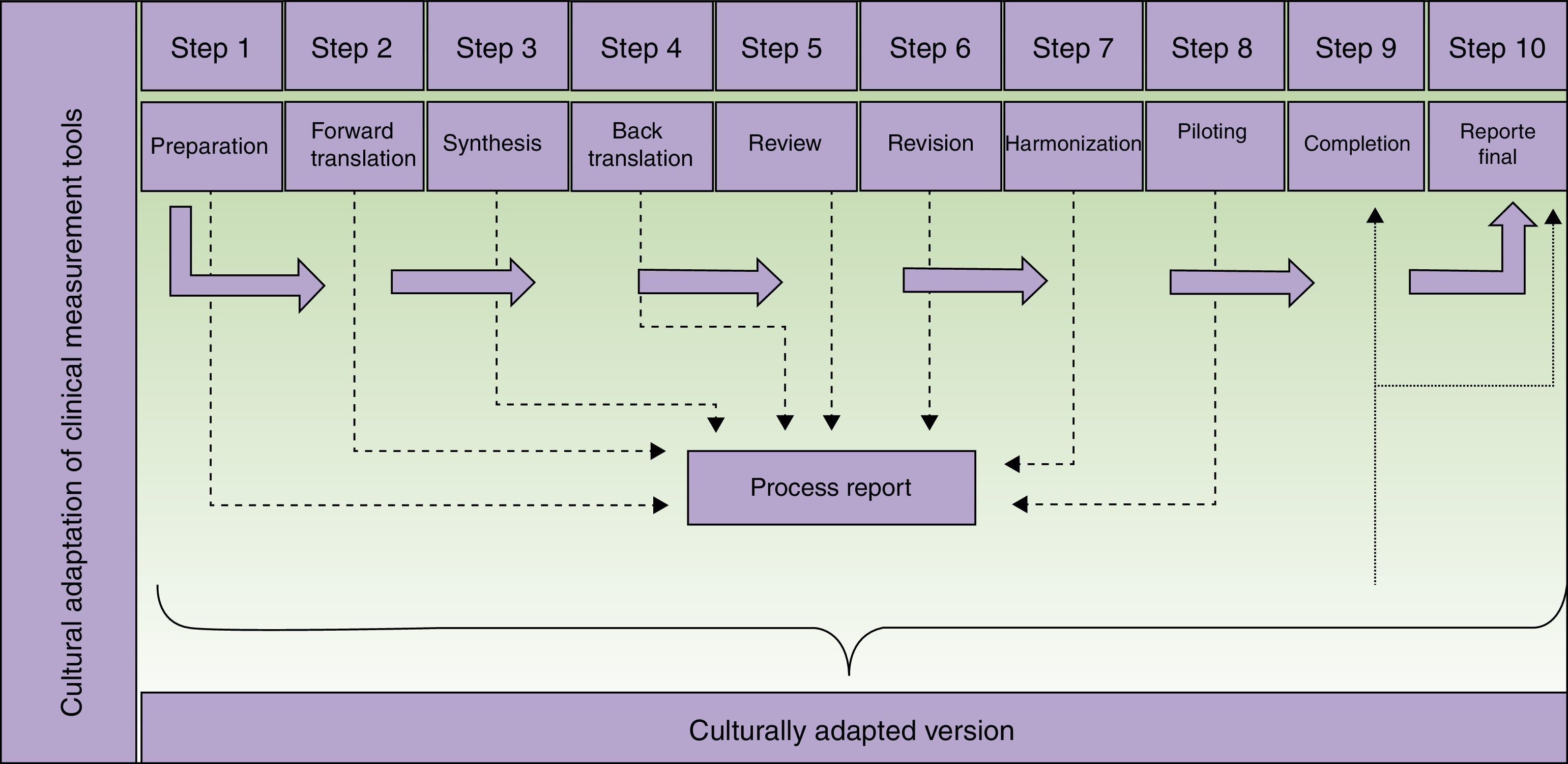
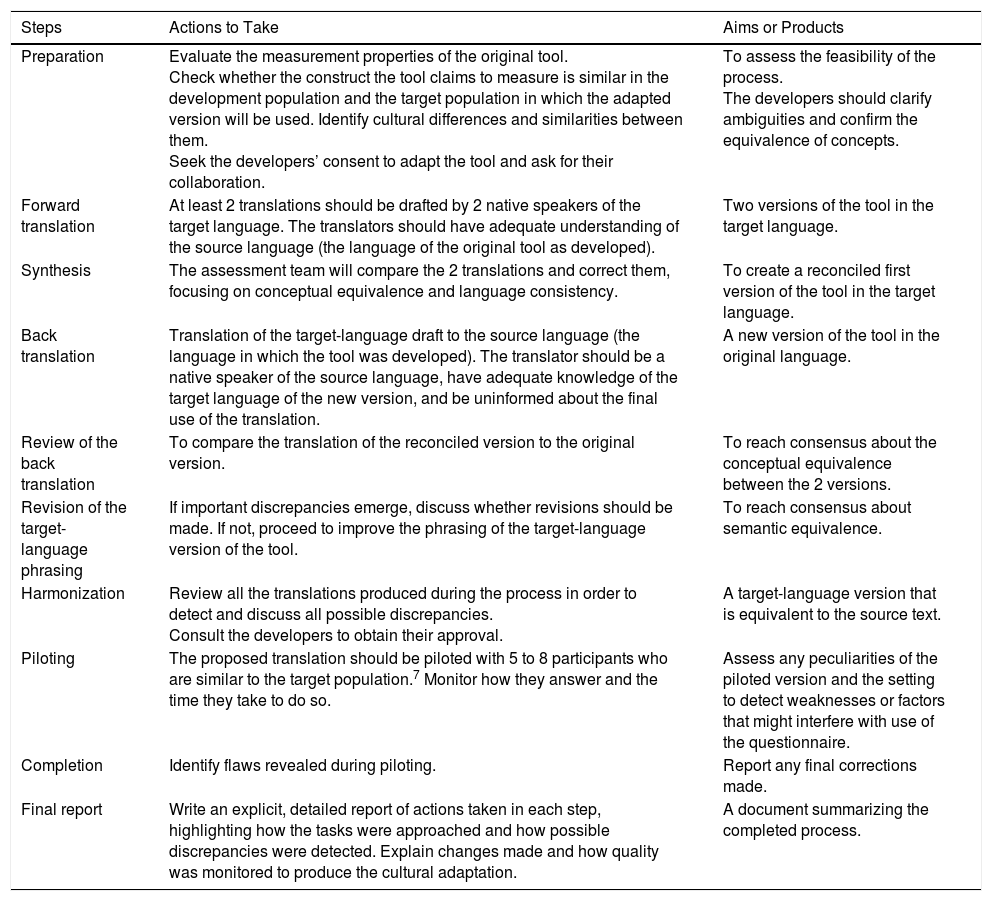
Each step of the cultural adaptation process calls for collecting theoretical and empirical evidence to support equivalence between the original and translated versions, thus underpinning the quality of the adaptation (Table 1).
Description of Steps to Follow When Translating and Culturally Adapting Health Assessment Tools and Producing a Final Report of the Process.
| Steps | Actions to Take | Aims or Products |
|---|---|---|
| Preparation | Evaluate the measurement properties of the original tool. Check whether the construct the tool claims to measure is similar in the development population and the target population in which the adapted version will be used. Identify cultural differences and similarities between them. Seek the developers’ consent to adapt the tool and ask for their collaboration. | To assess the feasibility of the process. The developers should clarify ambiguities and confirm the equivalence of concepts. |
| Forward translation | At least 2 translations should be drafted by 2 native speakers of the target language. The translators should have adequate understanding of the source language (the language of the original tool as developed). | Two versions of the tool in the target language. |
| Synthesis | The assessment team will compare the 2 translations and correct them, focusing on conceptual equivalence and language consistency. | To create a reconciled first version of the tool in the target language. |
| Back translation | Translation of the target-language draft to the source language (the language in which the tool was developed). The translator should be a native speaker of the source language, have adequate knowledge of the target language of the new version, and be uninformed about the final use of the translation. | A new version of the tool in the original language. |
| Review of the back translation | To compare the translation of the reconciled version to the original version. | To reach consensus about the conceptual equivalence between the 2 versions. |
| Revision of the target-language phrasing | If important discrepancies emerge, discuss whether revisions should be made. If not, proceed to improve the phrasing of the target-language version of the tool. | To reach consensus about semantic equivalence. |
| Harmonization | Review all the translations produced during the process in order to detect and discuss all possible discrepancies. Consult the developers to obtain their approval. | A target-language version that is equivalent to the source text. |
| Piloting | The proposed translation should be piloted with 5 to 8 participants who are similar to the target population.7 Monitor how they answer and the time they take to do so. | Assess any peculiarities of the piloted version and the setting to detect weaknesses or factors that might interfere with use of the questionnaire. |
| Completion | Identify flaws revealed during piloting. | Report any final corrections made. |
| Final report | Write an explicit, detailed report of actions taken in each step, highlighting how the tasks were approached and how possible discrepancies were detected. Explain changes made and how quality was monitored to produce the cultural adaptation. | A document summarizing the completed process. |
The availability of a research team willing to collaborate throughout the process is important. Each individual on the team should be assigned a specific role. Outsiders, such as translators or a review committee, will be necessary during some steps, and a supervisor should take charge of the process to ensure the proper method is followed.
Clinical measurement tools are usually under copyright and permission is required to produce an adapted version,1,4,10 although most authors make no mention of this requirement in their reports.
The translators who participate in the second step of the process (Fig. 1) should meet certain criteria. They should be experienced with or have some connection with the concepts to be assessed by the tool in order to decrease the likelihood that they will merely produce a literal translation. Professional translation service providers may be used. Their experience in translation will certainly provide a higher quality product, but professional status is not strictly necessary. It may even be possible for one of the translators to be the supervisor of the adaptation process, provided that person has the necessary language skills. If professional translators are contracted, we suggest they be briefed on the tool's use, target population, and the purpose of the translation—all in the interest of increasing final product quality.10
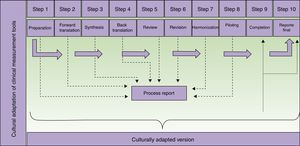
Steps in the translation and cultural adaptation of a clinical measurement tool. Adapted from Beaton et al.9
The third step requires the formation of a committee to evaluate and resolve discrepancies between the translations produced in the previous step. Members of the committee should have broad knowledge of the concepts being measured. However, they should not be familiar with the eventual proposed use for the adapted version, mainly because knowledge of the research objectives might cloud their judgment. Thus, the committee will involve persons who are not directly involved in the adaptation process itself. External service providers, such as translators or the developers of the instrument, if the latter are taking part, may join the committee.7 Approaches to building formal consensus, such as the Delphi method, are used to come to agreement. It must also be mentioned that 2 initial translations are sometimes insufficient. Should that be the case, additional ones might have to be commissioned.11
The revision of the back-translation in the fifth step should be undertaken by members of the team involved in the previous steps, although some guidelines mention the formation of a review committee. Deciding to form such a committee increases costs and prolongs the process.
The aim of the sixth step is to detect minor textual problems (such as grammar, typing or spelling mistakes) that might have been overlooked during the previous steps. The participants involved at this point can be the same ones who took part previously, including the translators.
Piloting the proposed adaptation has the purpose of evaluating its comprehensibility and operational equivalence. In this step any previously suggested alternative translations still under consideration can be revisited. Possibly inappropriate conceptual terms might be found in some items. Piloting seeks to identify any aspect that could generate confusion when the instrument is used. It is important to stress that this step focuses exclusively on evaluating the instrument, not analyzing the participants’ answers. Thus, the findings of this step are not what the patients or other subjects report in relation to each item, but rather their difficulty in responding and the amount of time they take to complete the questionnaire.
To carry out a useful pilot test of the translated tool, the team should consider and address the following points:
- •
The eventual target users (inclusion and exclusion criteria)
- •
Who will administer the questionnaire (if it is not a self-report instrument)
- •
The context in which the tool will be used
- •
Detailed instructions for how to register responses to the items
- •
How the results of piloting will be evaluated (generally with a qualitative approach)
The COSMIN checklist (Consensus-Based Standards for the Selection of Health Measurement Instruments) lists additional criteria, such as describing target users in terms of age, gender, disease characteristics, and source of recruitment.11
Once a pilot has been properly planned it must be carried out. Data gathered must be carefully analyzed by the working group so that they can detect specific problems that might require a return to one of the previous steps to review decisions taken earlier.
Any flaws detected should be discussed so that the participants can assess whether or not corrections to the structure or content of the instrument are warranted.
It should be mentioned that this adaptation process does not ensure that the new version will preserve the measurement properties of the original instrument. These properties might prove to have been compromised by changes made. Thus, a study that demonstrates the adapted tool's reliability and validity must still take place before clinical use can begin. Cultural adaptation of a measurement tool is a process that is distinct from its validation, even though the two processes are intimately related and each must carefully follow prescribed methods.4,10
The preparation of a final report is important because it reinforces the process that produces the adapted measurement tool and supports the resulting instrument. It will be even more important when it is used to gather research data. In each part of the process (Fig. 1) information is collected and must be analyzed in a step report, which will feed into the final report. In no case should the final report be skipped, as it can be used to guide future adaptation processes and is of interest to both researchers and clinicians.
One group published a report of their adaptation of the Dermatology Life Quality Index (DLQI) questionnaire to Spanish in 1998.12 The team consisted of a dermatologist, 2 specialized translators, a clinical psychologist, 3 clinical researchers, one of the DLQI developers, and an expert in health quality of life with wide experience in the cultural adaptation of instruments for measuring quality of life in dermatology. Their effort was noteworthy for its methodological rigor, and their report included a figure presenting the title and each of the 10 items in their original forms as well as the translations, the back-translations, and the level of equivalence achieved. We recommend that interested readers examine this material. Five patients piloted the questionnaire. Three had psoriasis and 2 had atopic dermatitis; their sociodemographic characteristics and levels of disease severity varied. We believe it is wise to include a larger number in a pilot test, even though the ideal number of respondents has ever been established. Depending on the level of objectivity reflected in the questionnaire being adapted and the target population in which it will be used, 20 to 30 patients might be advisable. The authors of the DLQI adaptation reported that the psychometric validation of the translated version would follow.
The reports of a validated translation and cultural adaptation of the Vitiligo-Specific Health-Related Quality of Life instrument (the VitiQoL) for use in Portugal13 and of the Nordic Occupational Skin Questionnaire (NOSQ-2002) for use in Spain and Catalonia14 included details of the validation studies of their instruments’ measurement properties. The VitiQoL report mentioned that the developers’ authorization had been obtained and that translators independently produced literal Portuguese versions of the original English items. These translations were revised by a group of bilingual health professionals, and 10 patients with vitiligo were asked to give opinions on the clarity and comprehensibility of the items. A revised version was then back-translated and sent to the developers. The results were not shown and the process was not discussed. The NOSQ-2002 adapters, on the other hand, did give a detailed description of their translation process. They included instructions given to the translators, analyses of each item, an account of the interviews that were required, corrections suggested by the developers, and more. The differences between these 2 reports seems to suggest that the VitiQol adapters underestimated the importance of the adaptation process, as they chose to provide more details related to the psychometric validation. The NOSQ-2002 adapters, on the other hand, detailed each process, in keeping with guidelines on good practice in translation and cultural adaptation, which consider it to be as important as the validation of measurement properties.7
The report of the adaptation and validation of the Spanish version of the Actinic Keratosis Quality of Life (AKQoL) questionnaire,15 originally developed in Danish, described the adaptation process in general terms but did not give details on how each step was handled. The adapters piloted their translated version in 38 individuals who were representative of the Spanish geographical area, but it seems they did not have actinic keratosis. The authors did not say whether they obtained the developers’ authorization or collaboration. The article focused on the validation of the translated version's measurement properties, even in the abstract, giving the impression that the authors underestimated the importance of adapted translation. They omitted including a report of the steps we have discussed here and summarized in Fig. 1.
A Spanish adaptation and validation of the Skin Cancer Index (SCI)16 was published in 2015. This instrument assesses quality of life in patients with nonmelanoma skin cancer on the face and neck. A committee of 3 dermatologists, a nurse and an epidemiologist was formed to assess the translation, and the first author of the original version participated actively in the process. The translation was piloted in 30 patients whose characteristics were not reported. The article includes the original version, the initially adapted Spanish version, and the Spanish version that later underwent validation. Once again the information given for the cultural adaptation process leaves us unable to evaluate the product, even though the study of the instrument's psychometric properties was described in detail.
In conclusion, as a large amount of space is required to explain the cultural adaptation of a measurement tool in detail, and given the importance of reporting that process, it would be best to publish the reports of cultural adaptation and the validation of measurement properties separately. Both carry equal weight and must be described explicitly and clearly.
Please cite this article as: Ortiz-Gutiérrez S, Cruz-Avelar A. Proceso de traducción y adaptación cultural de instrumentos de medición en salud. Actas Dermosifiliogr. 2018;109:202–206.