Gorlin syndrome is a rare autosomal dominant disease caused by mutations in the sonic hedgehog signaling pathway. Of particular importance is the PTCH1 gene. The disease is characterized by the development of multiple basal cell carcinomas at young ages. These tumors may present with other skin manifestations such as palmoplantar pits and with extracutaneous manifestations such as odontogenic keratocysts and medulloblastoma. Although the dermatologist may be key for recognizing clinical suspicion of the syndrome, a multidisciplinary team is usually necessary for diagnosis, treatment, and follow-up. Skin treatment may be complicated due to the large number of basal cell carcinomas and the extent of involvement. In recent years, new drugs that inhibit targets in the sonic hedgehog pathway have been developed. Although these agents appear promising options for patients with Gorlin syndrome, their efficacy is limited by adverse effects and the development of resistance.
El síndrome de Gorlin es una enfermedad infrecuente de herencia autosómica dominante producida por mutaciones en genes de la vía de señalización Sonic Hedgehog, entre los que destaca PTCH1. Se caracteriza por el desarrollo de múltiples carcinomas basocelulares en edades tempranas, que pueden ir asociados a otras manifestaciones cutáneas como pits palmoplantares, o a manifestaciones extracutáneas, entre las que destacan los queratoquistes odontogénicos y el meduloblastoma. El papel del dermatólogo es importante en la sospecha de este síndrome, pero suele ser necesario un equipo multidisciplinar en el diagnóstico, seguimiento y en el tratamiento de estos pacientes. El tratamiento dermatológico puede ser complicado debido al alto número de carcinomas basocelulares y a su extensión. En los últimos años se han desarrollado nuevos fármacos que inhiben la vía Sonic Hedgehog y parecen prometedores para estos pacientes, aunque su eficacia está limitada por los efectos secundarios y la creación de resistencias.
Gorlin syndrome, also known as nevoid basal cell carcinoma syndrome (Online Mendelian Inheritance in Man [OMIM]: 109400), is an autosomal dominant inherited disease that predisposes affected individuals to developmental defects and tumor formation, and multiple basal cell carcinomas (BCCs) in particular.1 The molecular pathogenesis of this syndrome is linked to the patched 1 gene (PTCH1), which encodes the PTCH1 transmembrane receptor, implicated in the sonic hedgehog (SHH) signaling pathway.2–4 Progress in therapy for these patients has been made recently with the introduction of the SHH inhibitor vismodegib, indicated for the treatment of recurrent or locally advanced metastatic BCC.5
EpidemiologyGorlin syndrome has a variable prevalence, according to published series, of between 1/30 8276 and 1/256 000.7 Farndon et al.8 established a minimum prevalence of this disease of 1/57 000 inhabitants, and estimated that 1 out of every 200 patients with 1 or more BCCs has Gorlin syndrome.
The life expectancy of patients with Gorlin syndrome is 73.4 years, which is significantly less than the general population, who have a life expectancy of approximately 80 years.9 The most frequent cause of premature death in these patients is medulloblastoma.10
Molecular PathogenesisGorlin syndrome is an autosomal dominant inherited disease, with high penetrance and variable expressivity.11 It is caused by loss of heterozygosity of the tumor suppressor gene PTCH1, which maps to chromosome 9q22.3.2PTCH1 forms part of the SHH signaling pathway, and so mutations in this gene lead to overexpression of the SHH pathway.4
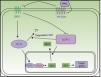
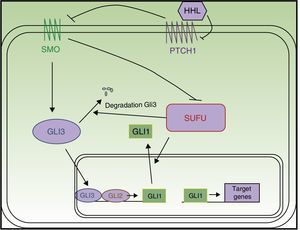
The SHH pathway was first described in Drosophila.12 It is essential for development, as it intervenes in tissue polarity and stem cell populations.13 In mammals, the pathway comprises 4 main elements (Fig. 1):
- 1.
Hedgehog ligands (HHL) of PTCH1: Sonic hedgehog, Indian hedgehog, and desert hedgehog
- 2.
PTCH1 receptor
- 3.
Smoothened (SMO) signal transducer
- 4.
Gli1, Gli2, Gli3 transcription factors13
PTCH1 constitutively inhibits SMO protein activity. Binding of HHL to PTCH1 suppresses inhibition of SMO by PTCH1. Once released, SMO translocates to the end of the primary cilium to exercise its function, with resulting activation of Gli transcription factors.14,15 The Gli proteins promote transcription of genes implicated in increased cell survival and mitosis.16 In vertebrates, there are 3 Gli proteins. Gli1 and Gli2 have an activation function, whereas Gli3 suppresses transcription of the target genes,14 including the Gli and PTCH1 genes. A relationship has also been demonstrated between the SHH pathway and other signaling pathways such as the epidermal growth factor, insulin growth factor, transforming growth factor beta (TGF-β), mammalian target of rapamycin (mTOR)/S6K1, protein kinase receptor C 1, notch, wnt/β-catenin, and phosphonositide-3-kinase (PI3K/Akt) pathways, such that all modulate cancer pathogenesis.4,13,14,17
PTCH1 is mutated in between 50% and 85% of patients with Gorlin syndrome,18,19 and these are de novo mutations in between 20% and 30%.20 Less frequently, mutations are found in other genes of the SHH pathway.21 Of particular note are the suppressor of fused (SUFU),22PTCH2, SMO, and GLI genes.23 The most frequently mutated gene after PTCH1 is SUFU, and this mutation should be investigated in patients with a genetic test negative for PTCH1.11 The presence of a SUFU deactivating mutation has been associated with lower penetrance and fewer major diagnostic criteria. Moreover, these patients have a higher risk of medulloblastoma and they do not present odontogenic keratocysts.22PTCH2 mutations are rare in patients with Gorlin syndrome, and these patients have a milder phenotype.23 The cases of somatic mosaicism produced by a mutation in the early phase of embryonic development are also uncommon.20
Most patients with Gorlin syndrome are born with a mutation inherited from one of the alleles of PTCH1, which is a null allele and encodes a truncated protein. For the disease to occur, the Knudson double hit phenomenon has to occur. The first hit or event is the inherited mutation and the second event corresponds to an acquired mutation in a healthy allele of the gene, which may occur due to external factors such as exposure to ultraviolet light, for example.21
Cutaneous and Extracutaneous Manifestations of Gorlin Syndrome: Diagnostic CriteriaTo date, no genotype-phenotype correlation has been established in these patients.11 Different classifications of diagnostic criteria have been reported for this disease, and these include both cutaneous and extracutaneous manifestations.20,24–28 In 2011, a consensus document was published with new diagnostic criteria in which molecular study was included for the first time (Table 1). In order to reach diagnosis of Gorlin syndrome, it is necessary to meet 2 major criteria, 1 major criteria and 2 minor criteria, or 1 major criterion with molecular confirmation.26 However, the sensitivity and specificity of the different proposals for diagnostic criteria have yet to be evaluated.11
Diagnostic Criteria for Gorlin Syndrome.
| Diagnostic Criteria |
|---|
| Major Criteria |
| BCCs in patients <20 years or excessive number of BCCs for solar exposure and phototype |
| Mandibular keratocysts in patients <20 years |
| Palmar/plantar pits |
| Lamellar calcification of the flax cerebri |
| Medulloblastoma |
| First-degree relative with Gorlin syndrome |
| Other skeletal abnormalities and radiologic changes (i.e. vertebral anomalies, kyphoscoliosis, short fourth metacarpals, postaxial polydactyly) |
| Minor Criteria |
| Rib anomalies |
| Macrocephaly |
| Ovarian or cardiac fibromas |
| Ocular abnormalities (i.e. strabismus, hypertelorism, congenital cataracts, glaucoma, coloboma) |
| Cleft lip or palate |
| Lymphomesenteric cysts |
Abbreviation: BCCs, basal cell carcinomas.
For diagnosis of Gorlin syndrome, it is necessary to meet 2 major criteria, 1 major criteria and 2 minor criteria, or 1 major criteria with molecular confirmation. Source: Bree et al.26
- -
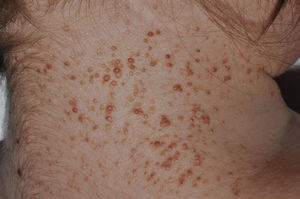
Basal cell carcinomas (Fig. 2). Basal cell carcinomas can appear early in life as multiple lesions.26,28 BCCs in Gorlin syndrome can involve both photoexposed and nonphotoexposed areas, with a slight predominance for photoexposed areas.1 The most frequent sites in men are the upper third of the back, arms, and the H zone of the face, whereas in women the lesions appear most frequently on the scalp, back, and legs. The proportion of each histologic subtype of BCC is similar to that of patients with sporadic BCC.29
- -
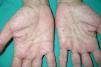
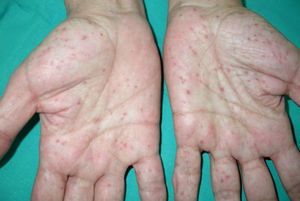
Palmar/plantar pits (Fig. 3). Palmar/plantar pits are a frequent manifestation and are reported in between 70% and 87% of patients.24,27 Between approximately 30% and 65% of patients with Gorlin syndrome present with palmar/plantar pits before they are 10 years old and most will have developed them by the time they are 15 years old.10 These lesions are punctate depressions, measuring 2-3mm across, that appear on the palms and soles and, more rarely, on the back or sides of the fingers and in interdigital folds.27 Histologically, these are areas of hypokeratosis with variable hypogranulosis, parakeratosis, and hyperplasia of basal cells with a palisade arrangement in the periphery.30
- -
Other skin manifestations. Multiple melanocytic nevi are frequent. A higher incidence of milium cysts on the lower eyelid and forehead and epidermoid cysts on the trunk have been reported.10
- -
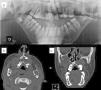
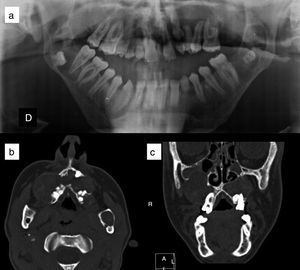
Mandibular or odontogenic keratocysts (Fig. 4). Such lesions are found in between 74% and 90% of patients with Gorlin syndrome.11,27 Onset may occur at ages between 4 and 5 years and they appear before 20 years in 75% cases27 but rarely develop in patients aged over 30 years. They are asymptomatic and are located on both sides of the jaw, and usually multiple lesions are present.10 Occasionally they undergo malignant transformation to ameloblastomas11 and squamous cell carcinoma.21
Figure 4.Odontogenic keratocysts in a patient with Gorlin syndrome. A, Panorex of the jaw, where keratocysts can be seen enveloping several molars. B, Axial computed tomography section in which maxillary odontogenic keratocysts can be seen. C, Coronal computed tomography section, showing maxillary keratocysts compressing the paranasal sinuses. Courtesy of Dr. E. García Esparza.
- -
Nontumoral manifestations of the central nervous system. The most frequent nontumoral manifestation of the central nervous system is calcification of the flax cerebri, present in 65% to 79% of patients.24,27,28 This finding is not detected in early infancy,31 but rather from adolescence onwards.28 Other rarer abnormalities are calcification of the flax cerebri, petroclinoid ligament, and diaphragma sellae.32
- -
Facial abnormalities. Relative macrocephaly and hypertelorism have been detected,27,33,34 often associated with telecanthus.27 A higher frequency of frontal, biparietal, or temporal bone protrusion has been reported.21
- -
Vertebral, rib, and shoulder blade abnormalities. Between 38% and 49% of patients have rib abnormalities,27,35 with the presence of bifid ribs being the most frequent.28 Other rib abnormalities include splayed, fused, absent, cervical, and rudimentary ribs. Congenital vertebral malformations are frequent, particularly scoliosis28,36 and bifid spinous process. Rare findings include occult spina bifida, hemivertebrae, elongated vertebral bodies, spondylolisthesis, and fusion of vertebral bodies.28 Congenital high scapula or Sprengel deformity is present in between 11% and 22% of patients.28,35
- -
Limb abnormalities. The most frequently reported limb abnormalities are flame-shaped radiolucencies in hand radiographs, present in 30% of patients. A higher rate of defects in hand and foot development syndactylia, polydactylia, and shortened fifth metacarpal have been reported.28
- -
Ocular and auditory abnormalities. In addition to hypertelorism, a higher prevalence of exophthalmus, rotatory nystagmus, internal strabismus, congenital cataracts, iris and choroid coloboma, and microphthalmia have been reported.10,24,37 Among otological abnormalities, of particular note is otosclerosis, conduction deafness, and posteriorly angulated ears.33
- -
Medulloblastoma. Malign brain tumor is more frequent in pediatric patients.38 Such lesions develop in 1% to 5% of patients with Gorlin syndrome24,27 as a result of alterations in the SHH pathway; often these lesions are the first manifestation of the disease.38 On average, they are detected at age 2 years, whereas in the general population, the age of onset is between 7 and 8 years.10,27,38 These medullobastomas have an intermediate prognosis, with overall survival rates between 60% and 80%.39 Standard treatment for medulloblastoma is surgery in combination with radiotherapy and chemotherapy. In patients with Gorlin syndrome, radiotherapy can lead to the appearance of BCC and other brain tumors at the irradiation site, and so it is necessary to identify these patients to optimize adjuvant treatment.38
- -
Cardiac and ovarian fibromas. Cardiac fibromas are uncommon, are present from birth or shortly afterwards,11 and can cause severe arrhythmias and heart failure.23 Ovarian fibromas are generally an incidental finding. They are asymptomatic and malignant transformation is uncommon, but they can impair fertility.11
- -
Other tumors. Meningiomas associated with Gorlin syndrome have been reported in up to 5% of patients,28 in some cases in an area treated with radiotherapy.27 Rarer types of tumor include head tumors such as astrocytoma, craniopharyngioma, and oligodendroglioma, as well as solid tumors and hematological malignancies,10 with fetal rhabdomyoma of particular note.23,40,41
There are few publications in the literature about which diagnostic tests should be performed in patients with Gorlin syndrome. The 2011 consensus document points to key data from the medical history, physical examination, and complementary examinations recommended for diagnosis and follow-up of these patients (Tables 2 and 3). It is important to minimize exposure to ionizing radiation and, if possible, the use of magnetic resonance imaging or ultrasonography is preferred.26
Medical History and Physical Examination in Patients With Gorlin Syndrome.
| Baseline Medical History and Physical Examination | Follow-up | |
|---|---|---|
| Medical History | Birth history: hydrocephalus, macrocephaly, undescended testes | |
| Psychomotor Development | ||
| Personal history: brain tumors, strabismus, cardiac problems, or infertility | ||
| Surgery: oral surgery or dental extractions, brain tumors, cleft lip or palate, skin excisions... | ||
| History of exposure to ultraviolet light or radiation | ||
| Physical Examination | Head and neck: measurement of the height-adjusted occipital-frontal circumference, frontal bossing, cleft palate, and dental malocclusion | |
| Skeletal examination: Sprengel deformity, scoliosis, pectus anomalies, and finger anomalies | ||
| Dermatological examination: basal cell carcinoma and palmar/plantar pits | Every year until onset of first basal cell carcinoma. Then at least once every 3 months | |
| Others | Neurology evaluation | Annually until 7-8 years old Annually in adults with a history of neuroblastoma |
| Ophthalmology evaluation | Annually in children. In adults, only in the event of symptoms | |
| Gynecology and urology evaluation | If symptoms present | |
| Baseline cardiac evaluation | If symptoms present | |
| Psychological Evaluation | Variable | |
| Analysis of the psychomotor development or learning difficulties | Variable | |
| Genetic Counselling |
Complementary Tests in Patients With Gorlin Syndrome.
| Baseline Complementary Tests | Follow-up | |
|---|---|---|
| Screening for Diagnostic Criteria | Plain radiography: ribcage, spine, hands, feet, pelvis (♀) | |
| Panorex of the jaw | At least annually from 8 years old or when the child is able to cooperate until first keratocyst. From then, every 6 months until 21 years old or 2 years without keratocysts. In adults, in the event of symptoms | |
| Gynecological ultrasound | Repeat if symptoms present | |
| Echocardiography | Repeat if symptoms present | |
| Genetic Study | ||
| Children | Head magnetic resonance imaging | Annually until 7-8 years old |
| Adults | Plain head radiography (from adolescence onwards) |
The genetic study is one of the major criteria proposed in the 2011 consensus.26 It is only possible to demonstrate PTCH mutation in approximately 60% to 80% of patients,20,21 and so it is recommended to reserve genetic study for 3 situations: in prenatal diagnosis if the family mutation is known; for confirmatory diagnosis in patients with some signs of the syndrome but who do not meet the diagnostic criteria; and as a predictive test in individuals at risk of presenting the disease, who do not meet the diagnostic criteria but who have an affected family member.26
Complementary Tests (Table 3)It is important to bear in mind the age of onset of the disease manifestations when choosing the most appropriate diagnostic tests.
Plain radiography of the ribcage and spine is recommended to rule out several of the minor criteria of the disease present from birth.27 Hand and foot radiography can also detect changes during childhood.28 Head radiography for diagnosis of calcification of the flax cerebri can be useful in adult patients,11,28 remembering that this finding is present from adolescence.
Odontogenic keratocysts appear from 4 years onwards, and so annual digital Panorex of the jaw is recommended in patients with suspected or confirmed diagnosis, from when the child is able to cooperate until appearance of the first keratocyst.26,27 Other authors recommend to start screening at 8 years old,11 with screening repeated every 6 months until the patient is 21 years old. In adults, Panorex of the jaw should be repeated annually if the patient presents symptoms.26
The risk of medulloblastoma is greatest between 2 and 3 years old, but this lesion can appear up until 7 years old. Neurological examination and brain magnetic resonance imaging are recommended until 7 to 8 years old. In adults with a history of medulloblastoma, baseline brain magnetic resonance imaging is recommended along with annual follow-up in the neurology department.26
Ovarian ultrasonography is recommended in asymptomatic women at menarche or at 18 years, or earlier if symptoms are reported.26,27 When Gorlin syndrome is diagnosed in adult women, baseline gynecological ultrasonography should be recorded.26 Prenatal counseling and follow-up of the pregnancy may be necessary for early detection of signs of the disease in the fetus.24,26 Cardiac fibromas are infrequent and asymptomatic, but an echocardiogram may be considered in the first year of life to detect them.11,26
In addition to the above tests, genetic counseling and psychological support may be necessary.26
PreventionThe preventive measures in patients with Gorlin syndrome include lifestyle changes and medical treatment. Patients are recommended to avoid exposure to ionizing radiation, sunscreen should be used rigorously, and vitamin D supplements are recommended.42 Retinoids have been proposed as systemic drugs for chemoprevention of BCC. The most widely used is oral isotretinoin, which should be administered at high doses for long periods to achieve the desired results, although this leads to more intense side effects. Moreover, the lesions recur once the drug is suspended.33,43 Chemoprevention with topical tazarotene has also been tested but no preventive or therapeutic effect found was found.44 Some authors have also suggested the use of photodynamic therapy (PDT)45,46 but the usefulness of this technique has not been studied.
Treatment of Basal Cell Carcinoma in Patients With Gorlin SyndromePatients with Gorlin syndrome receive a multidisciplinary treatment due to the different disease manifestations.11,26 This review focusses on the therapeutic modalities for BCC that are based on local control of the disease in these patients,47 with the aim of preserving function while providing the best possible cosmetic outcomes.48 The treatment modalities are divided into 3 groups: surgical treatment, nonsurgical treatment, and treatment based on molecular pathogenesis.
Surgical TreatmentConventional surgical excision is a commonly used method in patients with Gorlin syndrome but it has been little studied in the literature. To date, there are no studies of the outcomes of this technique or of recurrence rates in these patients. The safety margins recommended are of at least 4mm.48
Mohs micrographic surgery is indicated as treatment in Gorlin syndrome,21,49 particularly in high-risk recurrent tumors in the facial area or other areas of risk.50 With this technique, the cure rate at 5 years is 99% for primary BCC and 99.4% for recurrent BCC,51 but no data are available for patients with Gorlin syndrome.
The use of curettage and electrocoagulation as well as cryotherapy have been recommended in some cases of small primary, non-aggressive, BCCs that are histologically well defined in areas of low risk of recurrence such as the trunk or limbs.36,50,51 In these cases, the cure rate at 5 years is between 92% and 97% for sporadic BCC,48,51 but no studies have been performed in patients with Gorlin syndrome. Both options could be assessed in cases of multiple BCCs and extensive lesions.42
Cases have been published of BCC treated with ablative CO2 laser. This technique can treat superficial BCC in the same area.52–54
Nonsurigcal TreatmentPDT is a well-known and widely used treatment for BCC. In Europe, aminolevulinic acid and methyl aminolevulinate are used as photosensitizers.55 The rate of complete disappearance of sporadic superficial BCCs treated with PDT with methyl aminolevulinate was 92% to 97% at 3 months, with recurrence rates of 9% at 1 year and 22% at 5 years.56,57 PDT with methyl aminolevulinate is considered effective and safe in patients with Gorlin syndrome, and the technique can be used for the simultaneous treatment of multiple BCCs spread over large body areas. This treatment is recommended for superficial BCCs of any size and nodular BCCs with a thickness of less than 2mm.58
Topical chemotherapy with 5-fluorouracil (5FU) 5% is approved for the treatment of BCC in extrafacial, thin, low-risk tumors.59 In patients with Gorlin syndrome, the outcomes have been contradictory,60,61 although the treatment can be useful in combination with cyrotherapy62 or topical tretinoin.63 Likewise, there have been reports of a patient with Gorlin syndrome satisfactorily treated with oral capecitabine, a prodrug of fluorouracil, achieving regression of multiple BCCs and with good tolerance.64
Immunotherapy with imiquimod 5% in cream is approved for use in superficial BCCs. It is applied once a day, 5 days a week, for 6 weeks.59,65 It is not indicated in morpheaform BCC, infiltrative BCC, recurrent BCC, or when the site is the head.47 Sporadic BCC have shown a histological cure rate of 82% to 90% at 12 weeks,65,66 and a recurrence rate of 20.6% at 2 years.66 There have been reports of patients with Gorlin syndrome treated with imiquimod 5% for variable periods between 6 and 14 weeks, with good outcomes,67–70 but no long-term data are available.
In the literature, there are isolated reports of treatments with other therapeutic modalities, such as tretinoin 0.1% cream for treatment of extensively affected areas36 or ingenol mebutate in numerous lesions that have not responded to other therapies.71
Treatment Based on Molecular PathogenesisVismodegib (GDC-0449) acts on the SHH pathway, specifically inhibiting the SMO receptor. It was approved by the US Food and Drug Administration (FDA) for the treatment of metastatic or locally advanced BCCs that are not candidates for surgery or radiotherapy.5,72 In 2009, the first phase I trial (NCT00607724) was performed that evaluated the safety and pharmacokinetics of the drug at doses of 150mg/d, 270mg/d, and 540mg/d, establishing that the most appropriate dose was 150mg/d.73 The approval of vismodegib in 2012 was based on a multicenter phase II trial (ERIVANCE, NCT00833417), which demonstrated the efficacy of vismodegib at a dose of 150mg/d.74 In an updated analysis of the ERIVANCE trial, vismodegib attained an objective response in 48% of the patients with locally advanced disease and in 33% of the patients with metastatic BCC.75 Clinical trials have been conducted in locally advanced and metastatic BCC using a dose of 150mg/d of the drug (NCT01160250, STEVIE [NCT01367665], RegiSONIC [NCT 01604252]).76–78 Of those trials, a higher overall response rate was achieved in the STEVIE trial, which reported an objective response of 66.7% in patients with locally advanced BCC and 37.9% in metastatic BCC, with a median overall time to response of 2.7 months.77 The clinical trials conducted to date suggest that the efficacy and safety of vismodegib in patients with Gorlin syndrome with locally advanced and metastatic BCC are similar to those of patients with sporadic BCC.79 Vismodegib has also been shown to decrease the size of odontogenic keratocyts in patients with Gorlin syndrome.80
Recently, a new clinical trial (MIKIE [NCT01815840]) has been published. This trial assessed intermittent regimens of vismodegib therapy in patients with multiple BCCs (more than 6 tumors), including patients with Gorlin syndrome. Patients were randomized to treatment group A (vismodegib 150mg/d for 12 weeks, followed by 3 courses of 8 weeks placebo and 12 weeks treatment with vismodegib 150mg/d) or treatment group B (vismodegib 150mg/d for 24 weeks, followed by 3 courses of 8 weeks placebo and 8 weeks treatment with vismodegib 150mg/d). Both regimens achieved a decrease in the number of BCCs, although the decrease was greater in treatment group A in the subgroup of patients with sporadic BCC. Likewise, regimen A achieved a larger decrease in tumor diameter.81
Vismodegib has been studied as treatment and prophylaxis for BCC in patients with Gorlin syndrome, with use during prolonged periods. A double-blind phase II study was conducted (NCT00957229) in patients with Gorlin syndrome with at least 10 BCCs. In these patients, vismodegib significantly reduced the incidence of new operable BCCs compared with placebo, significantly reduced the size of pre-existing tumors, and reduced the number of surgical procedures required.82,83 Side effects are the main drawback of long-term treatment. These require suspension of treatment in many patients, resulting in tumor recurrence. Overall, 74% of patients in trial NCT00957229 had to interrupt treatment at some point due to adverse effects. Re-initiation of the drug in Gorlin syndrome was not associated with loss of efficacy although the side effects also recurred.83 For this reason, some authors propose intermittent treatment with vismodegib in Gorlin syndrome to achieve better tolerance of the side effects.84
Almost all patients in clinical trials have adverse effects, generally of low grade. Of particular note are muscle cramps, fatigue, dysgeusia, anorexia, alopecia, and weight loss. Between 22% and 32% of patients experience serious adverse events.74,75
In some cases, resistance to vismodegib develops due to acquired SMO mutations.85,86 These resistances are acquired quickly and can compromise the effectiveness of treatment.85 Likewise, an increase in keratoacanthomas and squamous cell carcinomas has also been reported in patients treated with this drug,14,87 due to secondary activation of the RAS/MAPK pathway when targeting the SHH pathway.87
Sonidegib (LDE225) is another SMO inhibitor approved by the FDA for patients with locally advanced BCC who are not amenable to surgery or radiotherapy.88 The pivotal study for this drug is the BOLT study (NCT01327053) which tested oral doses of 800mg/d and 200mg/d, and found similar efficacy in both cases. However, given the more favorable adverse effect profile with the 200mg/d dose, this dose is the recommended one. Most of the patients also experienced side effects. The most frequent are myalgias, dysgeusia, alopecia, nausea, weight loss, and creatine kinase elevations.89 Resistance to sonidegib can develop, and it appears that patients who develop resistance to vismodegib also fail to respond to sonidegib.90 In addition to oral administration, sonidegib has been used at 0.75% concentration in cream for patients with Gorlin syndrome, with promising results.91
Saridegib (IPI-926) is an orally administered cyclopamine derivative that selectively antagonizes the SMO protein. At doses of 160mg/d, it can also induce clinical response in patients with BCC, although the only clinical trial to date included just 39 patients with this disease.92
Itraconazole is a SMO inhibitor that prevents accumulation of this protein in the primary cilium.93 The drug has been tested at doses of 100mg/12h and 200mg/12h, achieving a reduction in tumor area of 24%.94 Arsenic and its derivatives also block accumulation of Gli2 in the primary cilium and impede SMO activation.95 Both itraconazole and arsenic trioxide, alone or in combination, inhibit the SHH pathway in vitro and inhibit tumor growth in murine models of medulloblastoma and BCC with mutated SMO resistant to other inhibitors of this protein.96
ConclusionsGorlin syndrome is an autosomal dominant inherited disease caused by mutations in the genes that encode proteins of the SHH pathway. The most important gene is PTCH1. The syndrome is characterized by the appearance of multiple BCCs along with other cutaneous and extracutaneous manifestations. A multidisciplinary team is therefore needed for diagnosis, follow-up, and treatment of these patients. Many treatment modalities are available for BCCs associated with Gorlin syndrome. Of particular note are conventional surgery, Mohs micrographic surgery, PDT, and topical imiquimod 5%. Recent studies with drugs such as vismodegib that inhibit the SHH pathway are opening up new therapeutic approaches in these patients, although in many cases, side effects and resistance limit their efficacy.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank Dr. Ángela Hernández Martín and Dr. Elena García Esparza for their help in drafting this article and providing photographs of their patients.
Please cite this article as: Palacios-Álvarez I, González-Sarmiento R, Fernández-López E. Síndrome de Gorlin. Actas Dermosifiliogr. 2018;109:207–217.