Mycosis fungoides (MF) — the most common cutaneous T-cell lymphoma — is a chronic, insidious disease characterized by frequent relapses. MF classically progresses from macules, plaques, and tumors to erythroderma. Multiple clinical variants have been described, and treatment varies according to clinical stage. Therapies target the skin in early stages, phototherapy being among the first-line options.1 When MF is refractory to first-line treatments or progresses to advanced stages, systemic therapies are used. Infections and secondary neoplasms — mainly in the skin, lungs, prostate, or colon — must be watched for.2
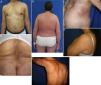
We describe a series of 41 patients with MF treated in the dermatology department of the University of Antioquia, Medellín, Colombia, between August 1, 2008, and March 30, 2011. Clinical and sociodemographic characteristics are presented in Table 1. Diagnoses were based on clinicopathologic correlations. We diagnosed classical MF in 18 patients and folliculotropic MF in 5. The remaining patients had clinical variants that are not recognized under the classification system of the Word Health Organization and the European Organisation for Research and Treatment of Cancer (WHO–EORT).3 The most frequent unrecognized variant we treated was hypopigmented MF, which was diagnosed in 24% of our patients (Fig. 1).
Sociodemographic Characteristics, Medical Histories, and Clinical Features.a
| Sociodemographic profile | |||
|---|---|---|---|
| Adult MF, N = 41 (100%) | Childhood MF, n = 7 (17.1%) | ||
| Age, y, median (min-max) | 48 (range, 11–86) | Age, y, median (min-max) | 14 (range, 11–18) |
| Ratio male:female | 1.1:1 | Ratio male:female | 1.3:1 |
| Medical history | No. of patients | Occupational history and exposures | No. of patients |
| Infections | 18 (44%) | Profession or workplace | |
| Labial herpes | 13 (32%) | Student | 8 (20%) |
| Herpes zoster | – | Homemaker | 8 (20%) |
| Chickenpox | – | Various | 7 (18%) |
| Pyoderma (>5 episodes/y) | 5 (12%) | Garment industry | 6 (15%) |
| Cancer | 4 (10%) | Agriculture | 5 (12%) |
| Lung cancer | 2 (5%) | Teaching | 3 (7%) |
| Basal cell carcinoma | 1 (2.5%) | Accountant | 1 (2.5%) |
| Carcinoid tumor of the colon | 1 (2.5%) | Secretary | 1 (2.5%) |
| Atopy | 17 (42%) | Sales | 1 (2.5%) |
| Rhinitis | 11 (27%) | Mining | 1 (2.5%) |
| Atopic dermatitis | 4 (10%) | ||
| Asthma | 2 (5%) | MF-associated exposures, >5 y | 25 (61%) |
| Chronic lichenoid pityriasis | 1 (2.5%) | Detergents | 7 (18%) |
| Alcohol consumption | 17 (42%) | Fumigants | 4 (10%) |
| Once a week | 8 (20%) | Chalk | 3 (7%) |
| Twice a week | 7 (18%) | Paint | 2 (5%) |
| Daily, until intoxicated | 2 (5%) | Paper | 2 (5%) |
| Current smoker | 7 (18%) | Amalgam (dental restorations) | 1 (2.5%) |
| Substance addiction | 1 (2.5%) | Mercury | 1 (2.5%) |
| Cocaine, heroin, | Lime | 1 (2.5%) | |
| benzodiazepines, glue | Fabrics | 1 (2.5%) | |
| Paper (industry) | 1 (2.5%) | ||
| Family history of MFa | Insecticides | 1 (2.5%) | |
| MF in 3 cases (uncle and nephew, 2 cases; cousin, 1 case) | |||
| Clinical characteristicsa | |
| Time until diagnosis, y, median (min-max) | 8.92 (0.4–40) |
| Skin phototypes | |
| I–II | 27% |
| III–IV | 64% |
| V–VI | 9% |
| Skin lesions | |
| Patches | 20 (49%) |
| Plaques | 12 (29%) |
| Tumors | 4 (10%) |
| Patches and plaques | 14 (34%) |
| Hypopigmentation | 10 (24%) |
| Folliculotropism | 5 (12%) |
| Erythroderma | 2 (5%) |
| Single lesion | 1 (2.5%) |
| Mean body surface area | 24.3% |
| T1, patches and plaques. <10% of surface | 24.3% |
| T2, patches and plaques, >10% of surface | 14 (34%) |
| Affected zones | |
| Head and neck | 22 (54%) |
| Arms | 16 (39%) |
| Trunk | 33 (81%) |
| Legs and buttocks | 32 (78%) |
| Genitals | 35 (85%) |
| Palms and soles | 4 (10%) |
| Adenopathy | 10 (24%) |
Abbreviation: MF, Mycosis fungoides.
Clinical characteristics of the most common presentations in the series: classical, folliculotropic, and hypopigmented MF. A, MF patches in a zone unexposed to the sun, with epidermal atrophy. B, MF presenting as an erythematous, infiltrated plaque. C, MF presenting as multiple, erythematous, ulcerated tumors. D, Folliculotropic MF with erythematous plaques and other lesions resembling epidermoid cysts. E, Folliculotropic MF with well-defined infiltrated plaques on the forehead. F, Hypopigmented MF with patches on the upper right arm. MF indicates mycosis fungoides.
Histologically, there was transformation to large cells in 1 case and to granulomas in another. The immune phenotype was available for 30 patients. The most common was CD3+ CD4+ CD7−. Double positivity (CD4+/CD8+) was found in 60% of the cases. Most patients had only skin involvement, lacking signs of metastasis or spread to lymph nodes. Palpable nodes were detected in 7 patients; 4 were biopsied, but MF was identified histologically in only 1 case. Radiographic staging was undertaken in all cases by means of a chest x-ray and abdominal ultrasound in accordance with current protocols. No visceral involvement was apparent. Biopsies ruled out bone marrow involvement in 5 patients. A complete blood count was ordered for all patients, but results were available for only 29 patients. A Sézary cell count in the extended peripheral blood panel for 21 patients showed percentages to be under 5% in all cases. The white cell counts were elevated in 5 patients. Lactate dehydrogenase levels were known for 18 patients (mean, 412 U/L; range, 207–556 U/L; reference range, 105–333 U/L), and 14 patients (78%) had elevated levels.
At the first clinical visit, MF was in an early stage (IA–IIA) in 87.8% of the patients. All underwent phototherapy (psoralen plus UV-A phototherapy in 40 patients, and narrowband UV-B phototherapy in 1 patient). A complete response was observed in 57% and a partial response in 24%. Seven also received systemic therapy: interferon was prescribed for 3 patients with folliculotropic MF; chemotherapy for the patients with tumors, transformation, or granulomas; and methotrexate for the patient with erythroderma. We followed 34 patients for a period ranging from 6 to 30 months. No disease progression was observed in 82%, but relapses after phototherapy were documented in 6 cases. Four patients died. Two died of infections (disseminated tuberculosis and sepsis) and 1 of brain cancer (glioblastoma multiforme). The cause was unknown in 1 case.
Several findings stand out in this patient series. The first is the median age of 48 years at presentation. That age is earlier than reported for the United States, Iran, and Japan (58, 52, and 55 years, respectively) but later than the median of 44.6 years reported for Singapore.4,5 We also observed high prevalences of infections, atopy, familial MF, and exposures to substances associated with higher risk for MF.6 The human leukocyte class II antigen alleles DR5, DRB1*11, and DQB*03 have been described in families with a history of MF.7
This case series had several clinical variants not included in the WHO–EORT classification. Hypopigmented MF was the most common, consistent with the findings reported for Singapore.8 This form of MF has a good prognosis and responds well to treatment. It is seen mostly in children, adolescents, and persons with dark skin (higher phototypes). We saw a considerable number of cases in children and adolescents, but our series had more individuals with light skin (lower phototypes).
MF is a great imitator of other dermatologic conditions.9 We observed presentations of MF with morphea (Fig. 2C) and hypopigmented MF associated with eruptive collagenomas (Fig. 2F) that have not previously been described in the literature. Collagenomas, which are connective tissue nevi, are seldom reported, possibly because of a dearth of epidemiologic studies on them. We found a single case report of papular elastorrhexis associated with Hodgkin lymphoma10 that was clinically similar to the collagenomas in our patients, suggesting these entities may share pathophysiologic features. We cannot say whether or not there is a causal relationship between MF and collagenoma formation; however, MF could possibly create a microenvironment on the skin that favors the development of these lesions, or the 2 conditions might have genetic or exposure factors in common.
Less common variants of MF. A, MF with transformations, presenting with plaques and tumors, some of which are eroded. B, MF with erythroderma; scaling erythematous plaques affect more than 80% of the surface. C, MF presenting as morphea with hyperpigmented sclerotic plaques on the thorax. D, Ichthyosiform MF with moderately sized dry scaly plaques in the lumbar region. E, Granulomatous MF with plaques and tumors, some of which are rounded and smooth while others are ulcerated. F, Hypopigmented MF with eruptive collagenomas. MF indicates mycosis fungoides.
Colciencias (file number, 111540820527) and the dermatology and internal medicine departments of the medical school of the University of Antioquia.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors express their thanks to the patients who agreed to participate in this study. We also thank the dermatology department of the University of Antioquia, and in particular Dr Marta Sierra Sierra for her review of some of the cases. We also thank the dermatopathology department and hematology laboratory of the university’s medical school. We acknowledge the support of Colciencias (file number, 111540820527).
Please cite this article as: Valencia-Ocampo OJ, Correa LA, Wolff-Idárraga JC, Velásquez-Lopera MM. Micosis fungoide, serie de 41 casos en Medellín, Colombia. Actas Dermosifiliogr. 2022;113:202–206.