Immunotherapy with programmed cell death 1 protein (PD-1) inhibitors is authorized by the European Medicines Agency for the treatment of locally advanced and/or metastatic cutaneous squamous cell carcinoma (cSCC).1 The only PD-1 inhibitor currently approved for use in this setting is cemiplimab. In Spain, however, neither this drug, nor any other PD-1 inhibitor, is covered by public funding for this indication, meaning that it is currently used off label or in expanded-use scenarios. We describe our experience with the use of PD-1 inhibitors in the treatment of locally advanced and metastatic cSCC in a dermatology department.
We undertook a retrospective observational study of patients treated with immunotherapy for locally advanced or metastatic cSCC in our dermatology department between January 2019 and January 2022. We gathered information on patient characteristics, tumor location and stage, previous treatments, PD-1 inhibitor treatment, response to treatment, time to response, disease-free time, immune-related adverse events (irAEs) classified using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0),2 and treatment of these events. We also studied PD ligand 1 expression (determined by immunohistochemistry), desmoplasia, and tumor infiltrating lymphocytes, evaluated using the modified Klintrup–Mäkinen score and classified as absent, mild, moderate, or marked.3
Seven patients (4 men and 3 women) with a median age of 85 years were identified (Table 1). Immunotherapy was indicated as first-line systemic treatment for metastatic cSCC in 1 patient and locally advanced cSCC in 6. All the cases were discussed by a multidisciplinary committee, and all the patients provided signed informed consent. PD-1 inhibitory treatment was monitored by the dermatology department.
Locally Advanced and Metastatic Squamous Cell Carcinoma Treated With PD-1 Inhibitors.
| All patients(n=7) | Complete response(n=5) | Stable disease/no response(n=2) | |
|---|---|---|---|
| Age, mean (range), y | 85 (81–91) | 85 (81–91) | 85.5 (84–87) |
| Female sex, No. (%) | 4 (57.1) | 3 (60) | 1 (50) |
| Treatment before PD-1 inhibitors, No. (%) | |||
| Surgery | 7 (100) | 5 (100) | 2 (100) |
| Radiation therapy | 4 (57.1) | 3 (60) | 1 (50) |
| Location of primary tumor, No. (%) | |||
| Head/neck | 5 (71.4) | 3 (60) | 2 (100) |
| Extremities | 1 (14.3) | 1 (20) | 0 (0) |
| Trunk | 1 (14.3) | 1 (20) | 0 (0) |
| Extent of disease, No. (%) | |||
| Locally advanced | 6 (85.7) | 4 (80) | 2 (100) |
| Regional metastasis | 1 (14.3) | 1 (20) | 0 (0) |
| Distant metastasis | 0 (0.0) | 0 (0) | 0 (0) |
| PD-L1 expression >1%, No. (%) | 2 (28.6) | 2 (40) | 0 (0) |
| Desmoplasia, No. (%) | 4 (57.1) | 2 (40) | 2 (100) |
| Tumor inflammatory lymphocytes, No. (%) | |||
| Absent | 1 (14.3) | 0 (0) | 1 (50) |
| Mild | 5 (71.4) | 4 (80) | 1 (50) |
| Moderate | 1 (14.3) | 1 (20) | 0 (0) |
| Marked | 0 (0) | 0 (0) | 0 (0) |
| ECOG score, No. (%) | |||
| 0 | 4 (57.1) | 3 (60) | 1 (50) |
| 1 | 3 (42.8) | 2 (40) | 1 (50) |
| Immune-related adverse events, No. (%) | |||
| Grade 1–2 | 3 (42.9) | 3 (60) | 0 (0) |
| Grade 3–4 | 1 (14.3) | 1 (20) | 0 (0) |
| Grade 5 | 1 (14.3) | 1 (20) | 0 (0) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PD-1, programmed cell death protein 1; PDL-1, PD ligand 1.
Five patients were treated off label with pembrolizumab 200mg every 3 weeks and 2 were treated with cemiplimab 350mg every 3 weeks in an expanded-use regimen. All the patients had been previously treated with surgery and 4 had also received radiation therapy. Five patients achieved a complete response and 1 did not respond. The disease stabilized in the other patient. Median time to response was 3 months and median disease-free time was 21 months (Table 2).
Overall Response to PD-1 Inhibitor Immunotherapy.
| All patients (n=7) | |
|---|---|
| Best overall response, No. (%) | |
| Complete response | 5 (71.4) |
| Partial response | 0 (0) |
| Stable disease | 1 (14.3) |
| Progression | 1 (14.3) |
| Time to response, median (range), mo | 1.5 (0–6) |
| Disease free time, median (range), mo | 21 (0–31) |
Abbreviation: PD-1, programmed cell death protein 1.
Immunotherapy was permanently discontinued in 3 patients due to a grade 4 irAE in 1 case and complete remission in 2. These 2 patients continued in complete remission 2 years after treatment discontinuation. All 3 patients in whom treatment was discontinued maintained a complete response, even a year after discontinuation. The disease-free time was longer than 20 months in all cases (21, 21 and 31 months).
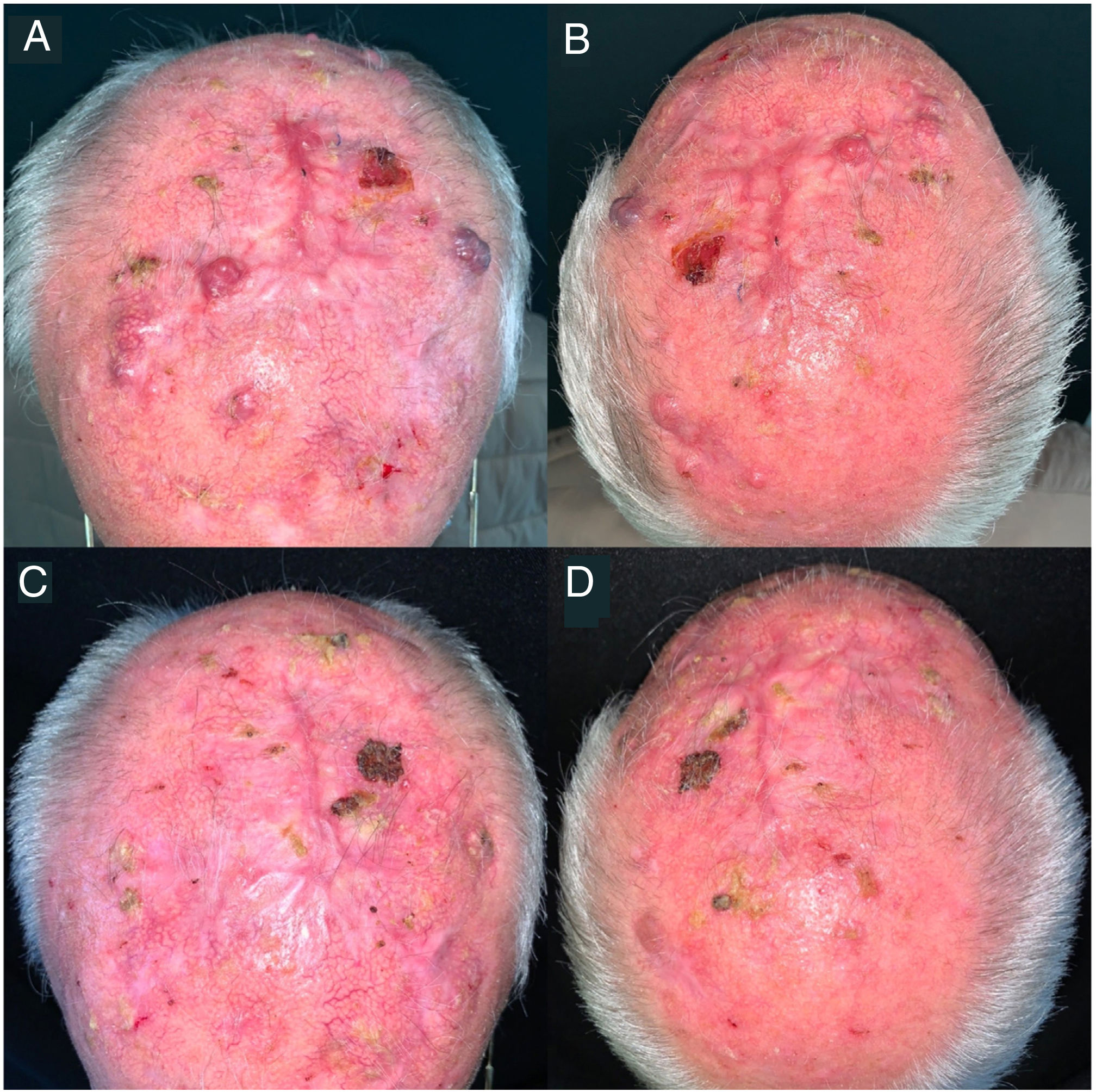
Five patients experienced irAEs, consisting of grade 1–2 rheumatic adverse events (polymyalgia rheumatica and arthralgia) that resolved with low-dose prednisone and/or hydroxychloroquine 200mg/d in 3 cases. Another patient developed grade 4 immune-mediated hepatitis during the third cycle of pembrolizumab; this was resolved by permanently discontinuing immunotherapy and starting the patient on methylprednisolone pulse therapy at a dosage of 250mg/d for 5 days and mycophenolate mofetil 1g/12h until complete resolution. The patient remained in complete remission without treatment up to the 21-month follow-up visit (Fig. 1). The fifth patient experienced a grade 5 irAE consisting of immune-mediated myocarditis.
Clinical images of a locally advanced, multifocal squamous cell carcinoma on the scalp. A and B, before immunotherapy. C and D, complete remission after a cycle of pembrolizumab 200mg every 3 weeks. The patient remained in complete remission after just 3 cycles of pembrolizumab and 21 months without treatment.
Three patients died: 1 due to disease progression, 1 due to a severe irAE, and 1 due to causes unrelated to the disease.
The efficacy and safety of PD-1 drugs in the treatment of locally advanced cSCC has been demonstrated in clinical trials for cemiplimab4 and pembrolizumab.5 These favorable outcomes have since been supported by real world data showing overall response rates of approximately 58% and complete response rates of 16%.6,7 Our study adds to real-world evidence on the use of immunotherapy in locally advanced and metastatic cSCC.
Five of the seven patients (71.4%) achieved a complete response. This notably high response rate might be related to the fact that none of the patients had received prior systemic treatment. It is similar to the higher response rates observed in patients receiving first-line immunotherapy in clinical trial settings.5 It is also notable that 3 patients maintained a complete response, even after more than a year without treatment. These patients could potentially be considered cured. Five of the 7 patients developed irAEs, which were serious in 2 patients (1 died as a result). All the complete responders developed irAEs, supporting the hypothesis that immune-mediated AEs might be linked to greater treatment effectiveness.
There is a need for effective treatments in the management of locally advanced and metastatic cSCC. PD-1 inhibitors offer a favorable profile (similar to that described in clinical trials) for use in this setting, even in elderly patients.
In conclusion, this series adds real-world evidence on the use of immunotherapy in locally advanced and metastatic cSCC. Dermatologists should be familiar with the characteristics, use, and potential adverse effects of these drugs.
Conflicts of InterestThe authors declare that they have no conflicts of interest.