A key aspect of the immune response alteration induced by systemic inflammation is the release of large numbers of pro- and anti-inflammatory mediators, mainly cytokines. However, data on similar changes in the skin, an organ that plays an important role in maintaining immune homeostasis, are scarce.
In this study, we used sexually mature C57BL/6 mice (30–35g; 12 weeks of age), which were divided into groups I [experimental; n=40], and II [control, n=10]). Mice in the experimental group underwent cecal ligation and puncture (CLP) to induce experimental sepsis, resulting in death within 3 days, after which control mice were sacrificed by administration of a high dose of anesthetic.1 Mice were housed in the animal facility, and all procedures were carried out in accordance with National Institutes of Health guidelines on the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Immunohistochemistry (IHC) was performed following the standard protocol using Bond-Max immunohistostainer (Leica, Germany) in automated mode. The following rabbit primary antibodies were used (Abcam, USA): anti-transforming growth factor (TGF) β, anti-interleukin (IL) 12, anti-IL-10, anti-IL-6, anti-CD206, anti-CD163, anti-CD68. Universal secondary antibodies (HiDefDetection™ HRPPolymersystem, Cell Marque, USA) were used. The number of positive cells, expressed as a percentage, was assessed in 10 randomly selected fields at ×400 magnification. The data obtained were processed using the statistical software package SPSS 7.5 for Windows (IBM Analytics, USA).
Mice in the experimental group died from sepsis during the first 3 days. Clinical signs of sepsis were observed in approximately 90% of mice during the observation period.
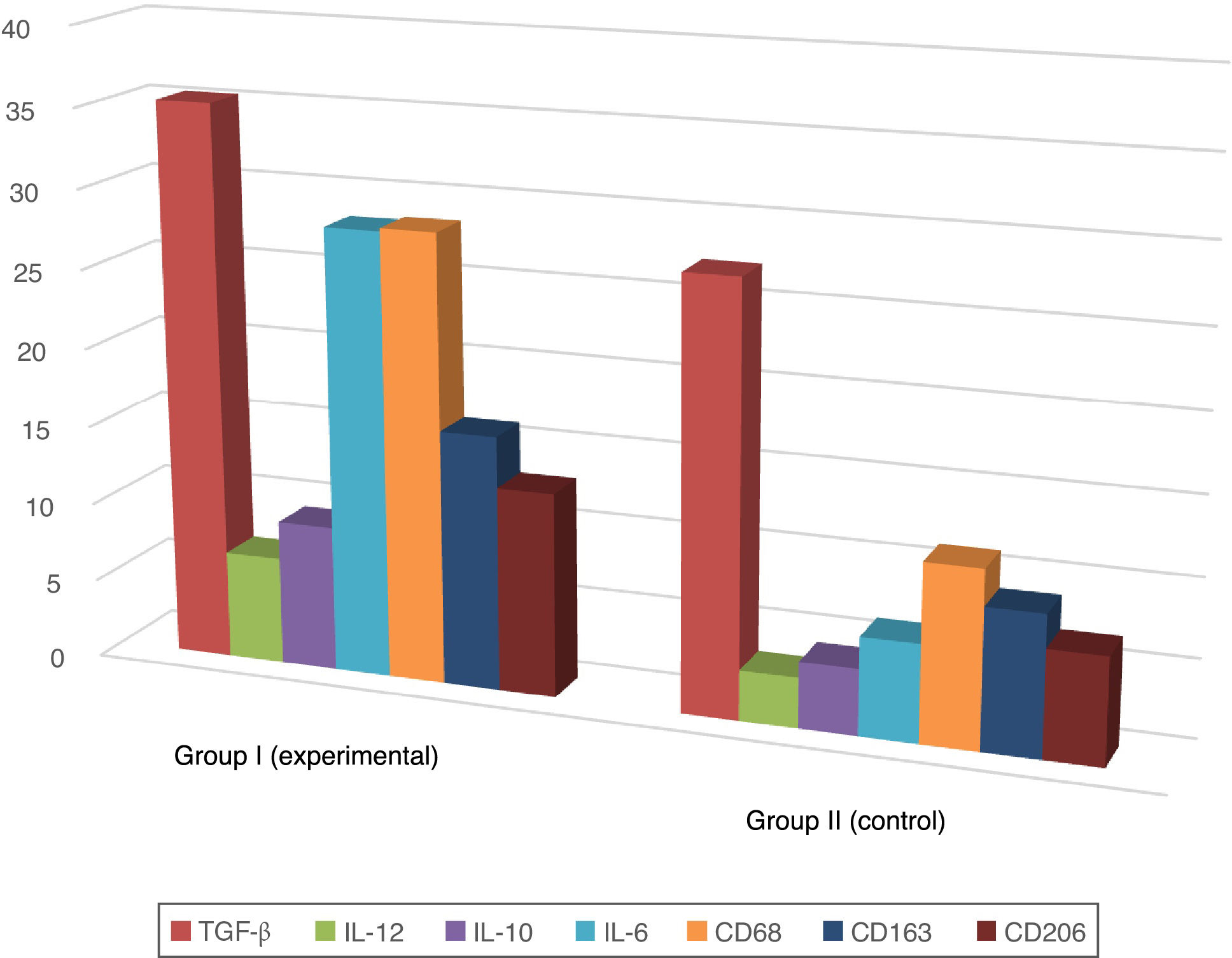
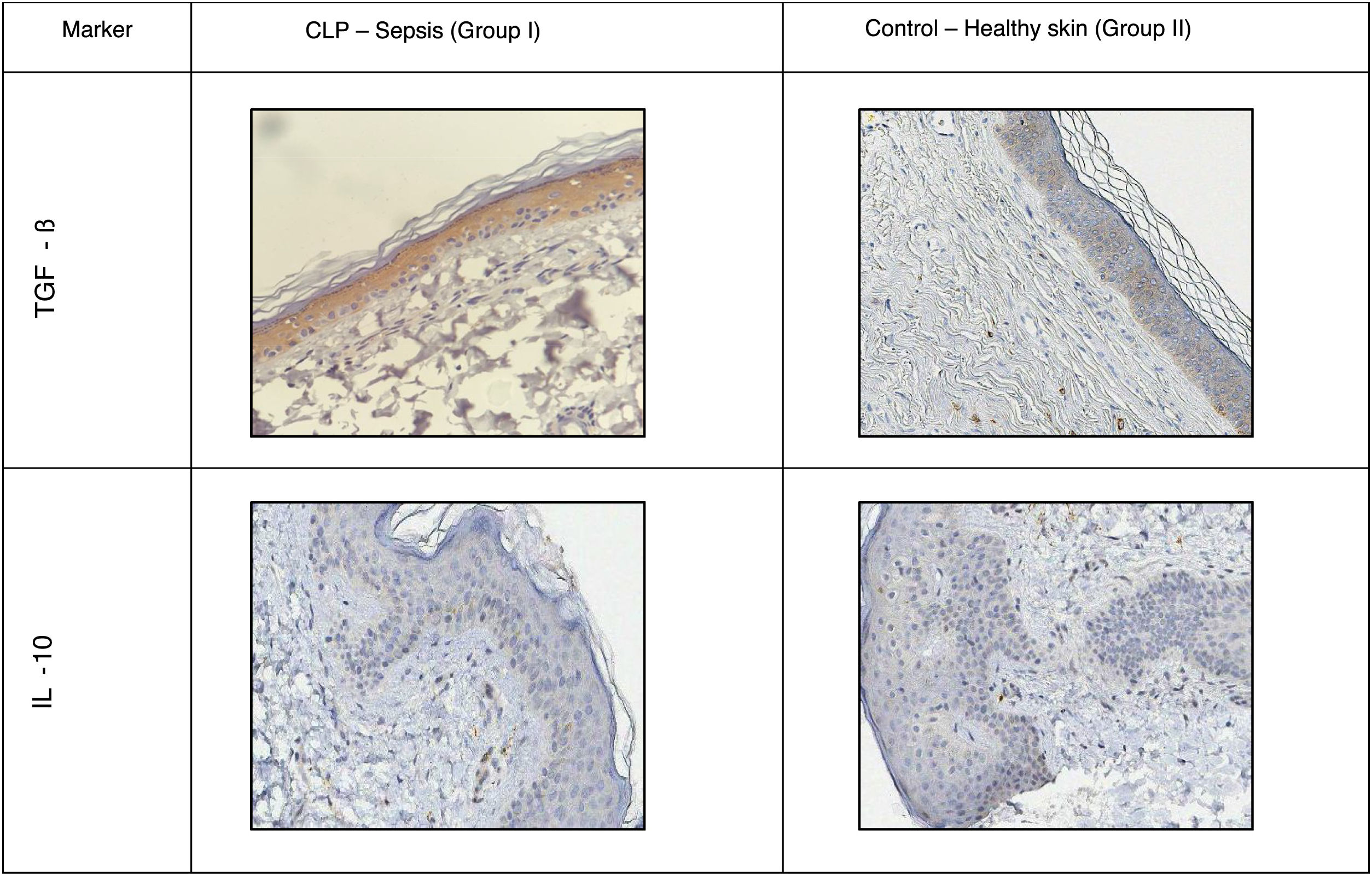
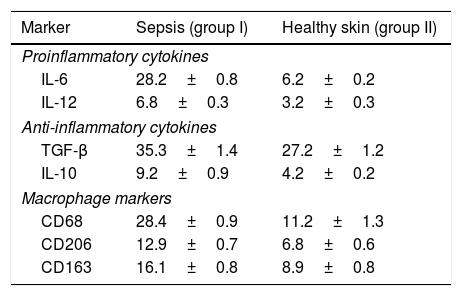
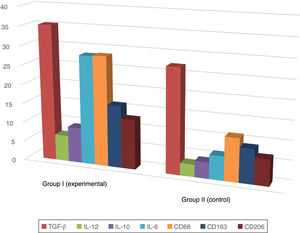
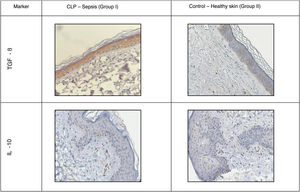
A positive immunohistochemical reaction to the aforementioned markers was detected in all skin samples studied, but the severity of sepsis was greater in the experimental versus the healthy control group (Table 1; Figs. 1 and 2).
Proportion of Immunopositive Skin Cells in Mice with Sepsis Versus Healthy Mice (%).
| Marker | Sepsis (group I) | Healthy skin (group II) |
|---|---|---|
| Proinflammatory cytokines | ||
| IL-6 | 28.2±0.8 | 6.2±0.2 |
| IL-12 | 6.8±0.3 | 3.2±0.3 |
| Anti-inflammatory cytokines | ||
| TGF-β | 35.3±1.4 | 27.2±1.2 |
| IL-10 | 9.2±0.9 | 4.2±0.2 |
| Macrophage markers | ||
| CD68 | 28.4±0.9 | 11.2±1.3 |
| CD206 | 12.9±0.7 | 6.8±0.6 |
| CD163 | 16.1±0.8 | 8.9±0.8 |
Abbreviations: IL, interleukin.
Distribution of TGF-β was predominantly intracellular. Control mice showed moderate staining in all layers of the epidermis and dermis, and a slight increase in hair follicles. Mice with sepsis showed increased staining intensity due to macrophage migration.
In the epidermis of mice with sepsis we observed the following fold increases in the proportion of interleukin-positive cells compared with the control group: IL-10, 2.2-fold; IL-6, 4.5-fold; IL-12, 2.2-fold.
Macrophage phenotyping also showed the following fold increases in the proportion of immunopositive cells in sepsis versus control mice: CD68, 2.5-fold; CD163, 1.8-fold; CD206, 1.9-fold. The distribution of CD163 remained predominantly intradermal, while CD68 and CD206 were detected in the dermis and epidermis.
It is hypothesized that the increase in proinflammatory cytokines in the skin during systemic inflammation may have a negative effect on the skin's defensive properties and lead to various diseases, both local and systemic. However, in our study we observed no morphological or functional skin disorders or signs of skin diseases, possibly because anti-inflammatory cytokines neutralize inflammatory effects.2,3
It is well known that TGF-β secreted by macrophages inhibits CD4 and CD8 T cells and some types of B cells, thus preventing the body's autoimmune response during inflammation.4 The observed increase in TGF-β immunostaining in the focus of inflammation is likely to be compensatory in nature and to occur in response to the increased number of proinflammatory mediators.
Macrophage differentiation towards the M1 phenotype is characterized by increased levels of proinflammatory interleukins (IL-6, IL-12) together with a predominance of CD68+ over CD206+ and CD163+ cells, an effect observed in the context of systemic inflammation in the present study. It is generally accepted that IL-6 activates a Th-2 mediated immune response, while IL-12 activates Th-1 differentiation, altering the function of the skin barrier.5,6 IL-10 inhibits T-cell reactivation, preventing massive autoimmune changes.7
Based on our results, it can be assumed that the epidermis participates in the general inflammatory process due to a change in the cytokine balance towards a predominance of proinflammatory mediators and M1 macrophages. However, it will be necessary to continue studying the interaction of these mediators, since skin dysfunction can result in increases in IL-6 and IL-12.8 We should also assume that destructive autoimmune changes occurring deeper in the skin are not detected, since in severe sepsis damage to vital organs occurs much faster and leads to multiple organ failure.
FundingNo public or private sources of funding.
Conflicts of InterestThe authors declare that they have no conflicts of interest.