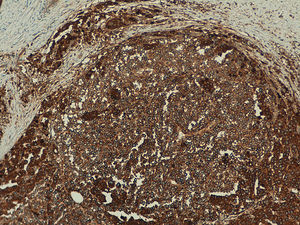
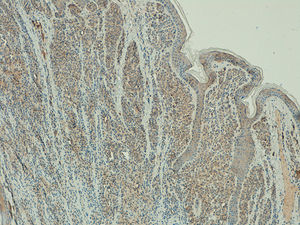
Telomerase is an enzyme involved in maintaining the length of telomeres and cell senescence. Numerous studies have shown that in more than 90% of malignant tumors telomerase activity is detected.
Material and methodsRetrospective observational study in a series of 85 cases of primary melanomas, 12 metastatic melanomas, and 22 melanocytic nevi. We used the monoclonal antibody hTERT (human telomerase reverse transcriptase, Rockland) to assess telomerase activity. The SPSS software package was used to analyze data.
ResultsTelomerase expression was present in all the melanocytic neoplasms analyzed. Expression was heterogenous and moderate or high in the melanomas. In contrast, expression was homogeneous and lower in the nevi. Heterogeneous expression was associated with rapid melanoma growth (P=.028), a Breslow thickness of more than 4mm (P=.004), mitosis (P=.032), and mutations in the TERT gene (P=.002). Activity was less intense in intradermal nevi, and more intense in compound and dysplastic nevi (P=.054).
ConclusionsTelomerase expression is found in all melanocytic neoplasms but is higher in melanomas than in nevi. A heterogeneous pattern of expression in melanomas is associated with more aggressive tumors.
La telomerasa es una enzima implicada en el mantenimiento de los telómeros y la senescencia celular. Numerosos estudios han demostrado que en más del 90% de las neoplasias malignas se detecta actividad telomerásica. El objetivo del presente estudio es analizar la expresión de telomerasa por inmunohistoquímica en una serie de neoplasias melanocíticas.
Material y métodosEstudio observacional retrospectivo realizado en una serie de 85 melanomas primarios, 12 metastásicos y 22 nevus melanocíticos. La expresión de telomerasa se analizó empleando el anticuerpo monoclonal hTERT (Rockland). El análisis de los datos se realizó con el programa SPSS.
ResultadosEn todas las neoplasias melanocíticas analizadas se demostró expresión de telomerasa. En el caso de los melanomas predominó el patrón de expresión heterogéneo, y la expresión moderada o intensa. En los nevus resultó más frecuente una expresión homogénea con intensidad leve. El patrón de expresión heterogéneo se asoció a los melanomas de rápido crecimiento (p=0,028), con Breslow > 4mm (p=0,004), con mitosis (p=0,032), y con mutaciones en el gen TERT (p=0,002). En el caso de los nevus, la intensidad fue menor en los nevus intradérmicos, seguidos de los compuestos y de los diplásicos (p=0,054).
ConclusionesLa expresión de telomerasa está presente en la totalidad de las neoplasias melanocíticas, con mayor expresión en los melanomas que en los nevus. En el caso de los melanomas, la expresión de forma heterogénea se asocia a un fenotipo de mayor agresividad.
Telomerase is an enzyme made up of a catalytic subunit (telomerase reverse transcriptase or TERT) and a ribonucleic acid (TERC).1 It is known to play a major role in telomere maintenance and cell senescence. Under normal conditions, it is only expressed in germline cells, fetal tissue, and stem cells. The somatic cells of other tissues lack telomerase activity, resulting in the progressive shortening of their telomeres after repeated cell division. Many studies have shown that telomerase activity is detected in more than 90% of malignant tumors,2 including different types of skin cancer.3 In melanocyte tumors in particular, several studies have identified increased telomerase activity in the neoplastic cells, from nevi to melanomas, with the highest telomerase activity detected in metastatic melanomas.4 Furthermore, the role of TERT promoter mutations in the pathogenesis of melanoma has recently been demonstrated.5,6 Several studies have identified recurring mutations in the TERT promoter region (c.1-146C>T, c.1-124C>T, c.1-124/-125CC>TT, c.1-138/-139CC>TT) in between 22% and 71% of sporadic melanomas associated with older patients, with melanomas in areas exposed to sunlight, of the nodular histologic subtype, a higher Breslow thickness, presence of ulceration, and high mitotic index.7–9 Studies in tissue and cell lines have shown that these mutations produce increased expression of telomerase by creating new binding sites for transcription factors of the ETS family.9–12 The presence of these mutations has been shown to be an independent prognostic factor linked to reduced disease-free and overall survival.8,13 Furthermore, this link to survival has been found to vary depending on the type of mutation identified, and is modified by the presence of rs2853669 polymorphism.14,15 Recent studies have also shown the prognostic value of telomere length by identifying lower survival rates in patients with shorter telomeres.16,17
It therefore appears that telomerase expression plays a major role in melanoma carcinogenesis; however, few studies have analyzed telomerase expression at the protein level, and those studies include small series with few melanomas. Moreover, the study of telomerase expression may be of utility for stratifying patients based on prognosis. The objectives of this study are to analyze telomerase expression by means of immune staining in a series of melanomas (primary and metastatic) and melanocytic nevi, and to correlate expression levels with the clinical and pathologic characteristics and the course of the patients, and with mutations in the TERT promoter.
Materials and MethodsSelected Patients and SamplesWe performed a retrospective observational study in the Dermatology and Anatomical Pathology Department of Hospital Universitario y Politécnico La Fe, Valencia, Spain, in a sample of 85 patients diagnosed with melanoma and 22 patients diagnosed with melanocytic nevus (5 with intradermal nevus, 7 with compound nevus, and 10 with dysplastic nevus), who were seen at our department between January 1, 2014 and December 1, 2016. Samples with sufficient quantity of sample in the paraffin block were selected for immune staining. In the case of primary melanomas, the only samples included were those with an invasive component, classified in 4 main histologic subtypes (lentigo maligna melanoma, surface spreading melanoma, nodular melanoma, and acral lentiginous melanoma), and those for which a study of the TERT promoter was available. The primary tumor was studied in the 85 patients with melanoma and melanoma metastasis was also studied in 12 of these patients. The clinical and pathologic data of each of the patients with melanoma was collected. Breslow thickness was characterized using the classification of the American Joint Committee on Cancer (AJCC) (≤1.00, 1.01-2, 2.01-4, and > 4mm)18; the mitotic index was expressed as the number of cells undergoing mitosis per square millimeter and was characterized by the presence or absence of mitosis; histologic regression was defined as described in previous studies,19 and was characterized as absent or present, and growth rate was defined as described in previous studies.20 All patients provided written consent to take part in the study.
Telomerase Expression With Immune StainingImmune staining was performed in 5-µm slices, using the hTERT monoclonal antibody (Rockland), as described previously.21 The same technique, with omission of the antibody, was used as a negative control. In the case of melanomas for which several blocks were available, those with the highest percentage of tumor cells were selected. Telomerase expression in all the samples included was performed by 2 independent observers (BU and RB). In the event of discrepancies in the observations of the 2 researchers, the observations of the researcher with most experience (RB) were used for the analysis. The extent of expression was quantified by establishing a score based on the percentage of positive cells (0, 0%; 1, < 10%; 2, 10%-50%; and 3, > 50%). The intensity of expression was scored from 0 to 3 (0, absent; 1, mild; 2, moderate; 3, intense). The expression pattern was classified as homogeneous or heterogeneous depending on the uniformity of expression in tumor cells.
Statistical AnalysisDescriptive analysis of the variables was performed using absolute and relative frequencies. Inferential analysis of the qualitative variables was performed using tests based on the χ2 distribution. Analysis of disease-free survival and overall survival was performed using the Kaplan-Meyer method. The degree of agreement between the independent observers was evaluated using the κ coefficient. Statistical analysis was performed using the SPSS statistical software package and statistical significance was established for values of p <0.05.
ResultsTelomerase Expression in Healthy SkinIn the healthy skin surrounding the melanocytic lesions, telomerase expression was observed in the epidermis, hair follicles, sweat glands, and sebaceous glands, and in the endothelium of the dermal blood vessels. Expression was mild in the epidermis and follicles, moderate in the sweat glands, and intense in the sebaceous glands (Fig. 1) (Figs. S1- S3 of the additional material available in the online version). Expression in these appendages was used as a positive control in all the analyzed cases.
Telomerase Expression in Melanocytic TumorsThe degree of agreement observed in the 3 parameters analyzed by the 2 researchers was good, with a κ index of 0.65 in intensity and 0.83 in extent, and 0.73 in telomerase expression pattern.
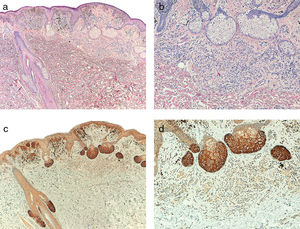
Telomerase was found to be expressed in all the melanocytic tumors analyzed. In the case of the primary melanomas, the expression pattern was homogeneous in all cell tumors in 59% (50/85) of cases (Fig. S4 of the additional material), whereas it was heterogeneous in 41% (35/85) of cases, with different expression intensity between tumor cells (Fig. 2) (Fig. S5 of the additional material). In most of the cases analyzed (95%; 81/85), expression was observed in over 50% of tumor cells (Fig. S6 of the additional material), and expression in less than 10% of the tumorous infiltrate was only observed in 1 case (Fig. S7 of the additional material). In terms of intensity, expression was intense in 41% (35/85) of the samples (Fig. S8 of the additional material), moderate in 51% (43/85) (Fig. S9 of the additional material), and mild in only 8% (7/85) of the samples (Fig. S10 of the additional material). Melanomas with a precursor nevus lesion (5%; 4/85) revealed intense telomerase expression in the tumor cells of the melanoma but mild expression in the accompanying nevus cells (Fig. 3A-3D).
A, Melanoma associated with intradermal nevus (hematoxylin–eosin, x40). B, Detail of intradermal nevus associated with the melanoma (hematoxylin–eosin, x100). C, Intense telomerase expression in melanoma tumor cells and mild expression in nevus cells (x40). D, Detail of mild telomerase expression in the nevus cells (x100).
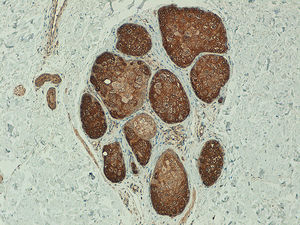
In the metastatic melanomas, the expression pattern was homogeneous in 67% (8/12) and heterogeneous in 33% (4/12). Expression of hTERT was observed in over 50% of tumor cells in 100% of the samples, and was intense in 41% (5/12), moderate in 50% (6/12), and mild in 8.3% (1/12) (Fig. 4).
Telomerase expression revealed a homogeneous pattern in 100% of the melanocytic nevi, with an extent of over 50% in the tumor cells in 91% (20/22) of the samples. Expression intensity was mild in 64% (14/22), moderate in 32% (7/22), and intense in 4% (1/22) of the samples. (Fig. 5).
In terms of differences in expression based on the clinical and pathologic characteristics of the primary melanomas, the heterogeneous expression pattern was associated with fast-growing melanomas (p=0.028) with a Breslow thickness of more than 4mm (p=0,004), with mitosis (p=0.032), and with mutations in the TERT promoter (p=0,002) (Table 1). The expression pattern showed no significant differences between the type of mutation in the TERT promoter (c.1-146C>T, c.1-124C>T, and c.1-124/-125CC>TT). No statistically significant differences were observed in intensity or extent of expression (Table S1 of the additional material). Expression intensity was higher in melanomas of the lentigo maligna melanoma subtype and lower in acral lentiginous melanomas, although this difference was not statistically significant (p=0.523). Analysis of survival revealed no statistically significant differences based on telomerase expression.
Clinical and Pathologic Characteristics of Patients With Melanoma and Distribution of Characteristics Based on the Telomerase Expression Pattern.
| Variable | Total (n = 85) | Expression pattern | ||
|---|---|---|---|---|
| Hom | Het | p | ||
| N (%) | N (%) | N (%) | ||
| Age | ||||
| < 40 | 5 (5.9) | 2 (40.0) | 3 (60.0) | 0.678 |
| 40-65 | 45 (52.9) | 27 (60.0) | 18 (40.0) | |
| > 65 | 35 (41.2) | 21 (60.0) | 14 (40.0) | |
| Sex | ||||
| Male | 46 (54.1) | 29 (63.0) | 17 (37.0) | 0.526 |
| Female | 39 (45.9) | 21 (53.8) | 18 (46.2) | |
| Location | ||||
| Head and neck | 9 (10.6) | 2 (22.2) | 7 (77.8) | 0.025 |
| Torso | 45 (52.9) | 32 (71.1) | 13 (28.9) | |
| Upper limbs | 14 (16.5) | 5 (35.7) | 9 (64.3) | |
| Lower limbs | 14 (16.5) | 9 (64.3) | 5 (35.7) | |
| Acral | 3 (3.5) | 2 (66.7) | 1 (33.3) | |
| Histologic subtype | ||||
| Lentigo maligna melanoma | 3 (3.5) | 0 (0) | 3 (100) | 0.017 |
| Surface spreading melanoma | 64 (75.3) | 43 (67.2) | 21 (32.8) | |
| Nodular melanoma | 15 (17.6) | 5 (33.3) | 10 (66.7) | |
| Acral lentiginous melanoma | 3 (3.5) | 2 (66.7) | 1 (33.3) | |
| Breslow thickness | ||||
| < 1 mm | 40 (47.1) | 29 (72.5) | 11 (27.5) | 0.004 |
| 1-2 mm | 29 (34.1) | 15 (55.2) | 13 (44.8) | |
| 2-4 mm | 6 (7.1) | 4 (66.7) | 2 (33.3) | |
| > 4 mm | 10 (11.8) | 1 (10.0) | 9 (90.0) | |
| Ulceration | ||||
| No | 69 (81.2) | 43 (62.3) | 26 (37.7) | 0.141 |
| Yes | 16 (18.8) | 7 (43.8) | 9 (56.3) | |
| Mitosis/mm2 | ||||
| < 1 | 43 (50.6) | 30 (69.8) | 13 (30.2) | 0.032 |
| ≥ 1 | 42 (49.4) | 19 (47.6) | 22 (52.4) | |
| Regression | ||||
| No | 43 (50.6) | 23 (53.5) | 20 (46.5) | 0.215 |
| Yes | 42 (49.4) | 27 (64.3) | 15 (35.7) | |
| Tumor stage | ||||
| Local | 73 (85.9) | 43 (59.7) | 29 (40.3) | 0.580 |
| Local-regional/distant disease | 12 (14.1) | 7 (58.3) | 5 (41.7) | |
| Growth rate | ||||
| Slow growth | 70 (82.3) | 25 (64.3) | 45 (35.7) | 0.028 |
| Rapid growth | 15 (17.7) | 5 (33.3) | 10 (66.7) | |
| Clinical course (%) | ||||
| Stable disease | 69 (81.0) | 41 (57.7) | 30 (42.3) | 0.242 |
| Local-regional/distant relapse | 11 (13.0) | 3 (42.9) | 4 (57.1) | |
| Death | 5 (6.0) | 3 (100.0) | 0 (0) | |
| Mutations in TERT promoter | ||||
| WT | 61 (71.8) | 37 (66.1) | 19 (33.9) | 0.002 |
| Mutated | 24 (28.2) | 6 (27.3) | 12 (72.7) | |
Het indicates heterogeneous; Hom, homogeneous; WT, wild type.
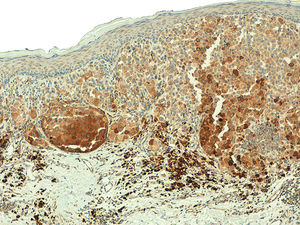
The comparative analysis of nevus with primary and metastatic melanoma revealed that telomerase expression in the nevi was less intense than in the primary and metastatic melanomas (p=0.001) and that, unlike the melanomas, the nevi revealed a characteristic pattern of homogeneous expression in all the tumor cells (p < 0.001) (Fig. 6). No statistically significant differences were observed between primary and metastatic melanomas. Moreover, in the case of the nevi, intensity was lowest in the intradermal nevi, followed by the compound and dysplastic nevi, although the differences were not statistically significant (p=0.054).
DiscussionThe results of this study show that telomerase expression occurs in all the melanocytic tumors analyzed, and that a gradually increasing trend from nevus to melanoma exists, which may be implicated in the tumor progression in this type of cancer.
In the primary melanomas in our series, we showed that the heterogeneous expression pattern is associated with more aggressive melanomas (faster-growing melanomas with a tumor thickness of more than 4mm, with mitosis, and with mutations in the TERT promoter); this has not been previously reported. These results contrast with those recently reported by Hugdahl et al,22 who describe homogeneous expression in most of their cases. Our findings, however, may support those reported by Kohli et al,23 who report heterogeneous expression of non-nuclear telomerase in melanomas but not in benign lesions. This heterogeneous expression pattern may be the result of intratumoral heterogeneity in melanoma, which has been linked to different tumor subpopulations, and is linked to resistance to systemic treatments.24,25 Furthermore, we cannot rule out the possibility that the heterogeneity observed in our study may be due to a defect in the staining procedure, although this is unlikely, as this pattern was not observed in any of the benign melanocytic lesions.
Unlike other studies, in our series, the intensity and extent of telomerase expression were independent of the clinical and pathologic characteristics. Hugdahl et al22 reported a statistically significant link between telomerase expression (44% of primary melanomas in that series) and tumor thickness, and Zygouris et al26 described a link with Breslow thickness and ulceration. Our findings contrast with several studies that have analyzed telomerase activity, such as the study by Ramírez et al, which found greater telomerase activity in melanomas with greater tumor thickness, a higher Clark score, and mitosis27; the results of Miracco et al,28 in which telomerase activity was also associated with Breslow thickness and the Clark score; and the study by Carvalho et al,29 which found greater telomerase activity in ulcerated melanomas, with a greater Breslow thickness, mitosis, vascular invasion, and satellitosis. In our series, melanomas of lentigo maligna melanoma showed greater expression intensity, although this did not reach statistical significance. This last finding does not support the findings of the series by Pópulo et al,8 in which telomerase expression was only associated with the surface spreading histologic subtype.
With respect to the mutations in the TERT promoter, in line with reporting by other authors,8,9 in a previous publication,30 we reported a prevalence of 33% and an association with ulcerated melanomas with greater tumor thickness, with mitosis, and of the nodular histologic subtype. As in other studies, in this series, we found no differences using immune staining in telomerase expression between mutated and nonmutated melanomas.8,22,23 This finding contrasts with several prior publications, which found greater telomerase expression in melanomas with mutations in the promoter.9–12 This discrepancy may be due to the fact that those studies analyzed telomerase expression at the messenger RNA level and not at the protein level, and other molecular mechanisms may therefore exist that regulate telomerase expression. Indeed, several studies have focused on epigenetic regulation of telomerase expression. It has been reported that post-transcriptional regulation of the TERT through microRNA expression may suppress telomerase expression31,32 and that hypermethylation in the DNA of the TERT gene may increase telomerase expression.33 It may therefore be useful to measure the length of the telomeres and/or telomerase activity and correlate it to the presence of the mutation in order to determine whether only mutated genes show increased activity or whether wild-type genes also show greater telomerase activity through these other molecular mechanisms.
Furthermore, we have identified variable levels of telomerase expression in a series of 22 melanocytic nevi with mild expression in most of the samples, and a tendency for expression to increase from intradermal nevi to dysplastic nevi. It should also be noted that, in all the melanomas with a nevus precursor, telomerase expression in the accompanying nevus was much less intense than in the tumor cells of the melanoma. These results suggest a potential role of telomerase in tumor progression in melanocytic cancers. Several authors have analyzed telomerase activity in different types of melanocytic tumor and have found that telomerase expression increases progressively from common nevi to dysplastic nevi and melanomas.4,34 These differences in telomerase expression between nevus and melanoma, however, have been found not to be of use in the differential diagnosis, as expression may be variable in both types of tumor.30
In this study, telomerase expression was analyzed using immune staining, unlike most previously published studies, which analyzed telomerase activity using the telomere repeat amplification protocol (TRAP).4,23–25 Immune staining has the advantage of being a simple and cheap method that is performed using paraffinized samples and makes it possible to visualize the intensity and extent of expression, not only in the cells of interest but also in adjacent healthy skin. Indeed, in line with what has already been described,30 we have identified variable levels of telomerase expression, not only in the tumor cells but also in the epidermis, hair follicles, glands, dermal blood vessels, and even in the accompanying inflammatory infiltrate.
In conclusion, the results of this study show that telomerase expression occurs in all the analyzed melanocytic tumors, with greater expression in the melanomas than in the nevi. In the case of the primary melanomas, heterogeneous expression is linked to a more aggressive phenotype; however, expression intensity is independent of the clinical and pathologic characteristics of the tumors. It has also been shown that no differences exist in telomerase expression based on the mutational state of the TERT gene and other molecular mechanisms must therefore exist that regulate expression of the TERT gene in the melanomas.
FundingThis study was carried out with grants awarded by Fundación Piel Sana (EUROMELANOMA 2016 grant from the AEDV Foundation), Carlos III Institute of Health (ISCII) (PI16/01559), IISLaFe (2014/0370), and the Department of Education, Research, Culture and Sport of the autonomous government of Valencia (GV/2016/064).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bustos BdU, Torralba AS, Poveda PM, Simó GP, Farinos JS, Ros ML, et al. Estudio de la expresión de telomerasa en una serie de neoplasias melanocíticas. Actas Dermosifiliogr. 2019;110:212–219.