A Spitz nevus is a melanocytic neoplasm of epithelioid and/or spindle cells that usually appears in childhood. These lesions are by nature benign, but their features can sometimes make them difficult to distinguish from melanomas. Spitzoid melanocytic lesions have been grouped into 3 types in recent decades: Spitz nevi, atypical Spitz tumors, and spitzoid melanomas. Atypical Spitz tumors are spitzoid melanocytic proliferations that have atypical histopathologic features that are insufficient to support a diagnosis of melanoma. The malignant potential of these lesions is at present uncertain. This review examines the clinical, dermoscopic, and histopathologic features of this group of lesions.
El nevo de Spitz es una neoplasia melanocítica de células epitelioides y/o fusiformes que suele aparecer en la infancia. Su naturaleza es benigna, aunque en ocasiones puede mostrar unas características difíciles de distinguir del melanoma. En las últimas décadas se han clasificado las neoplasias melanocíticas spitzoides en tres tipos: nevus de Spitz, tumor de Spitz atípico y melanoma spitzoide. El tumor de Spitz atípico hace referencia a las neoplasias melanocíticas spitzoides que tienen unas características histopatológicas atípicas insuficientes para realizar el diagnóstico de melanoma y cuyo potencial maligno, actualmente, es incierto. Nuestro objetivo es revisar los aspectos clínicos, dermatoscópicos, histopatológicos e inmunohistoquímicos de este conjunto de tumores.
A Spitz nevus is a melanocytic neoplasm formed of epithelioid or spindle cells described for the first time by Darier and Civatte.1 It was characterized in 1948 by Spitz using the term juvenile melanoma as a melanocytic tumor, present in childhood, with clinical and pathologic features similar to melanoma but with benign behavior.2 Subsequently, it was found to affect all age groups.3 From its first description until present, the lesion has been a controversial entity given its similarity to melanoma and the lack of consensus to establish either its diagnostic features or a strategy for clinical management.
Melanocytic neoplasms with a spitzoid morphology encompass a range of behaviors from benign to malignant. Over the last few decades, there has been a tendency to classify these lesions in 3 types, which are at times are hard to differentiate from one another: conventional Spitz nevus, atypical Spitz tumor, and spitzoid melanoma. This concept of intermediate category, denoted atypical Spitz tumor or of uncertain malignant potential, refers to those histological lesions with insufficient atypical characteristics to make diagnosis of melanoma.4–6 This category has been subject to much criticism. Some authors believe that there should be a clear distinction between benign and malignant tumors, and they affirm that the introduction of this term has led to confusion about the meaning of biologic behavior.7 However, recent articles suggest that many pathologists accept this concept of intermediate lesions8 and that there is a range of genetic mutations in these lesions (see part 2).
EpidemiologyThe overall incidence of Spitz nevi is not well documented, but it is thought to lie between 1.4 and 7 cases per 100 000 persons per year.9–11 These lesions represent fewer than 1% of melanocytic tumors that are excised during childhood.12
Spitz nevi generally appear during childhood or in young adults, although they can affect all age groups. They are extremely uncommon in individuals aged over 30 years.10 Congenital cases, present at birth or presenting in the first 24 months of life, are exceptional.13 One study of 349 patients found that 40% of lesions appear in children under 15 years and 77% present at ages below 30 years.14 The risk that a lesion with a spitzoid appearance is in fact melanoma increases with increasing age, whereas the probability that it is a Spitz nevus decreases.
There is generally no difference in incidence by sex, although between 15 and 30 years, there is a slight predominance in females (3:1). It has been suggested that this difference could be associated with a possible hormonal influence.3,12,15,16
Spitz nevi are more common in whites than in Asian or African individuals.16,17
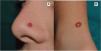
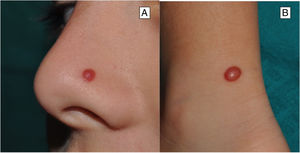
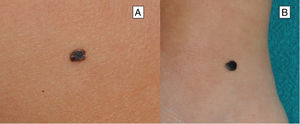
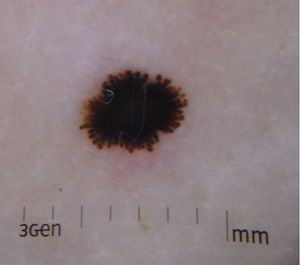
Clinical FeaturesSpitz NevusSpitz nevi generally appear in childhood as a solitary well-defined, dome-shaped papule or mass, with a solid consistency and a color ranging from flesh color, pink, red, to brown (Fig. 1). The color, often reddish, is due to the low melanin content and high vascularization, although 10% of lesions are pigmented (Fig. 2).3 The lesions grow rapidly for a period of 3 to 6 months and, although their size is generally less than 5-6 mm, they can reach a diameter of 1-2 cm. After this phase of rapid growth, the lesion can remain static for years, showing a progressive transformation until acquiring the aspect of a common melanocytic nevus or involving until completely disappearing.18,19 In adults, the lesion is usually pigmented with a color between brown and black.
Although the lesion can be located anywhere, including the mucosae, in children there is a predilection for the head and neck (37%), whereas in young adults it is preferentially located on the lower limbs (28%).20,21
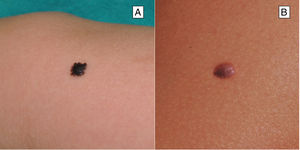
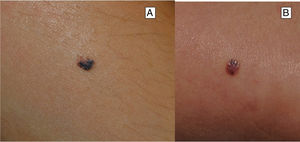
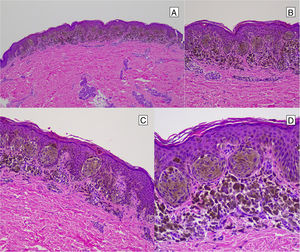
Reed Nevus or Pigmented Spindle-Cell NevusReed nevus was described in 1975 as a benign, highly pigmented, melanocytic lesion, with a dark or black color and a size generally less than 8 mm (Fig. 3).22 These lesions usually present on the legs of older children and adolescents, more frequently on the thighs in young women and on the trunk in adults.23 Some authors consider that Reed nevus is a subtype of Spitz nevus whereas others consider it as an independent entity. Detection may have increased with the introduction of dermatoscopy.18
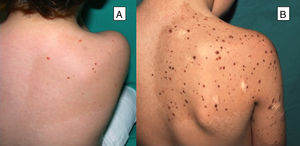
Multiple Spitz NeviMultiple Spitz nevi are characterized by the presence of 2 or more Spitz nevi in the same patient. This presentation is uncommon and, depending on the distribution of the lesions, the condition can be divided into 2 subgroups: grouped or disseminated.24 Multiple grouped Spitz nevi can develop over a café-au-lait spot25 (Fig. 4), on normal skin, or, less frequently, on a hypopigmented background. When multiple Spitz nevi appear with a disseminated pattern, if they develop over a short period of time, they are known as multiple eruptive Spitz nevi. Their etiology is unknown, although it has been postulated that their appearance could be related to a genetic predisposition or trigger factors. Cases have been reported after sunburn, perioperative stress, pregnancy, drug abuse, Addison disease, chemotherapy, and allogeneic hematopoietic stem cell transplantation.24
Desmoplastic Spitz NevusDesmoplastic Spitz nevus is a rare subtype, more common in individuals in late adolescence or in adults than in children. Clinically, lesions present as papules with limited or no melanocytic pigmentation, poorly defined borders, and woody consistency. They are most frequently located on the limbs.26
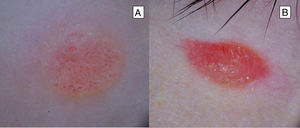
Atypical Spitz TumorAtypical Spitz tumors are usually larger (> 10 mm) than classic Spitz nevi and, unlike the latter lesions, are usually asymmetric, generally with irregular borders, and the surface may be ulcerated3,6 (Table 1) (Fig. 5). In a retrospective study of 72 spitzoid neoplasms in patients aged between 1 and 18 years, 7% were found to be atypical Spitz tumors.27
Clinical Features of Conventional Spitz Nevi and Atypical Spitz Tumors.
| Clinical Features | Classic Spitz Nevus | Atypical Spitz Tumor |
|---|---|---|
| Age | < 10 years | > 10 years |
| Site | Face, neck, and extremities | Trunk |
| Size | < 10 mm (generally 5-6 mm) | > 10 mm |
| Borders | Well-defined and regular | Poorly-defined and irregular |
| Surface | Smooth | Irregular, ulcerated |
| Color | Pink, reddish | Irregular |
Adapted from Luo et al.3
Melanoma is uncommon in children; however, most melanomas diagnosed in children are spitzoid melanomas. These are an uncommon variant of melanoma that, in pathology study, may resemble Spitz nevi. In general, these are usually amelanotic or pigmented lesions with different colors that show a progressive growth; they can grow to more than 1 cm in diameter, often with ulceration. The lesions are usually located on the head, neck, or limbs. Their biological behavior is similar to conventional melanoma, although more favorable outcomes have been observed in spitzoid melanomas in children under 10 years of age or prepubescent children.28,29
DiagnosisDiagnosis of Spitz nevus can be difficult given its similarity with other skin tumors and differential diagnosis is necessary (Table 2).
Main Differential Diagnoses for Spitz Nevi.
| Conventional or hypopigmented Spitz nevus | Pyogenic granuloma |
|---|---|
| Mastocytoma | |
| Hemangioma | |
| Angiofibroma | |
| Juvenile xanthogranuloma | |
| Molluscum contagiosum | |
| Amelanotic melanoma | |
| Pigmented Spitz nevus | Congenital and acquired melanocytic nevus |
| Melanoma | |
| Desmoplastic Spitz Nevus | Keloid |
| Dermatofibroma | |
| Multiple Spitz Nevi | Metastatic melanoma |
The introduction of dermatoscopy has enabled greater diagnostic precision for Spitz nevi given the identification of a variety of patterns that provide additional information about their morphology (Table 3).30,31 Although the identification of these patterns has contributed to improved tumor detection, none of them are specific to Spitz nevi and they can also be present in melanoma, and so careful interpretation is of great importance.32
Dermatoscopic Patterns Present in Spitzoid lesions.
| Pattern in Pigmented Spitzoid Lesions | Description |
|---|---|
| Starburst pattern | Central pigmentation with homogeneous greyish, blueish, and black color with regular radial projections (streaks or pseudopods) at the periphery |
| Globular pattern | Central blue-black pigmentation and presence of regularly distributed round or oval structures of brown-black color at the periphery |
| Homogeneous pattern | Diffuse, brown, grey-blue, or grey-black pigmentation in the absence of other structures |
| Atypical or multicomponent pattern | Uneven distribution of colors and structures |
| Reticular pattern | Pigment network similar to that present in acquired melanocytic nevi |
| Reticular depigmentation | Network of hypopigmentation (white lines surrounding pigmented globules) |
| Pattern in Hypopigmented Spitzoid Lesions | Description |
|---|---|
| Punctiform vascular pattern | Dotted vessels with regular distribution |
| Glomerular vascular pattern | Tortuous or coiled capillaries |
| Vascular pattern with hairpin vessels | Looped or bending vessels |
| Vascular starburst pattern | Regular radial vascular lines at the periphery |
| Homogeneous pinkish color | Pinkish tone with or without remnants of brownish pigmentation in absence of other structures |
| Reticular depigmentation | Whitish network surrounding the vessels |
| Chrysalis structures | Linear, orthogonal, or disordered lines of bright white color. These can be seen with dermatoscopy under polarized light, associated with the previous pattern |
Adapted from Lallas et al.31
Classic Spitz nevi have been more frequently associated with the presence of a dotted vascular pattern. This consists of dotted and monomorphic vessels, distributed regularly over a pinkish background (Fig. 6). This pattern is present in 51% of Spitz nevi. In pigmented Spitz nevi, a starburst or globular pattern is more frequently observed. This pattern is characterized by a homogeneous central pigmentation with colors ranging between greyish, blueish, and black, with radial projections at the periphery in the case of the starburst pattern (Fig. 7) and round or oval structures of brown-black color, distributed regularly at the periphery in the case of a globular pattern; these patterns are present in 51% and 17% of Spitz nevi, respectively.30,31,33 Another pattern, initially associated with melanoma, but which is present also in Spitz nevi, is the reticular depigmentation pattern, which consists intersecting lines surrounding the pigmented globules or vessels.32 This latter pattern may present in association with a dotted or comma vascular pattern, or the globular pattern.34,35 It is interesting to highlight that if the globular pattern is not associated with reticular depigmentation, it is not characteristic of Spitz nevi, as these regular round or oval structures at the periphery are frequently observed in melanocytic nevi in children.27,36 Other patterns, such as the homogeneous one, which is characterized by brown or black pigmentation in absence of other structures, or the reticular pattern, in which a network of pigment is observed similar to other acquired melanocytic nevi, may be present in the natural course of Spitz nevi. A sequential course has been described in which Spitz nevi may go through a series of different dermatoscopic patterns, with a progressive transformation from a globular pattern to a starburst one, and even to a homogeneous pattern before involution and spontaneous disappearance in more than 50% of cases.18,37–39 The multicomponent pattern is characterized by an uneven distribution of colors and structures.33,40 In a study that assessed the dermatoscopic features of atypical Spitz tumors, it was observed that most lesions showed this pattern and 16.4% showed an overall dotted vascular pattern.41
Although dermatoscopy is a useful technique, it is not always sufficient to differentiate between Spitz nevus and melanoma. The symmetry of the arrangement of structures and colors throughout the lesion is characteristic of the spitzoid pattern and should suggest to us diagnosis of Spitz nevus, whereas asymmetry, presence of a multicomponent pattern, or visualization of chrysalis, although potentially present in Spitz nevi, force us to consider melanoma.31 In a case-control study conducted in children in which dermatoscopic features were compared according to age group (0-6, 7-12, and 13-18 years), it was observed that the vascular and globular patterns were more prevalent in preschool children, whereas the starburst or multicomponent pattern predominated in children of school age, and a reticular depigmentation pattern was observed more frequently in adolescents.27 However, a systematic review that included 15 case-control studies and case series with patients of all ages, but with more adults than children, concluded that the type of pattern was not associated with the age of the patient.31
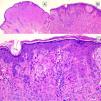
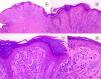
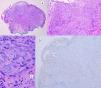
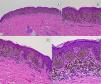
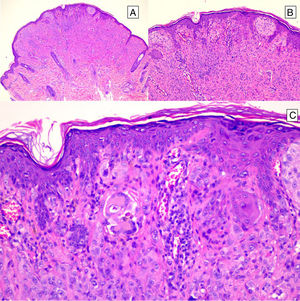
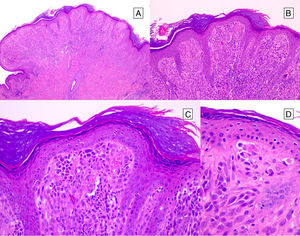
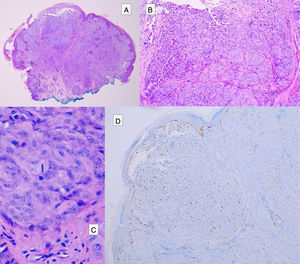
HistopathologySpitz NevusSpitz nevi are symmetric, well-delimited, melanocytic lesions, generally with a compound presentation although like other melanocytic nevi, they can be located at the dermal-epidermal junction or within the dermis. They are characterized by the presence of epithelioid (rounded or polygonal) or spindle-shaped melanocytes with enlarged regular nuclei with peripheral margination. These show abundant cytoplasm and prominent central nucleoli. The cells are distributed in the form of uniform nests with a vertical orientation and, occasionally, a pagetoid spread of isolated melanocytes can be present at the uppermost layers of the epidermis or the junction. The dermal component matures with depth and, to a lesser extent, towards the periphery. The epidermis can be preserved or show regular hyperplasia; the dermal vessels are usually dilated and clefts can be seen among the melanocyte nests.14,41–44 In the epidermis or papillary dermis, there is often a presence of eosinophilic globules, which contain proteins of the basal membrane; these structures are also known as Kamino bodies.45 They are characteristic of Spitz nevi, but they can also be present in melanoma, although in this latter case, they are not usually very big or well formed.46 Lymphocytic inflammatory infiltrate can also be observed in the base and perivascular region. Typical mitosis can also occasionally appear (generally less than 2/mm2) in the mid or upper part of the lesion; this finding is rare in the deep dermis and atypical mitosis is not observed. The pigment can be granulated and located in the superficial part of the lesion14,41–44 (Table 4) (Figs. 8–10).
Clinical Features of Conventional Spitz Nevi and Atypical Spitz Tumors.
| Spitz Nevus | Spitzoid Melanoma |
|---|---|
| Symmetry | Asymmetry |
| Well delimited | Poorly delimited |
| Intact or hyperplastic epidermis | Epidermis can be ulcerated |
| Kamino bodies | Absence of Kamino bodies |
| Maturation with depth | Absence of maturation with depth |
| Limited pagetoid spread | Extensive pagetoid spread |
| Fewer than than 2 mitosis/mm2 | Frequent mitosis in the dermis; these can be atypical |
| Low nucleus/cytoplasm ratio | High nucleus/cytoplasm ratio |
| Nuclei increased in size, but absence of nuclear pleomorphism | Cellular pleomorphism, high degree of cytological atypia |
Intradermal Spitz nevus. Although most Spitz nevi are compound lesions, a small portion of them can be limited to the dermal-epidermal junction. Cytological features, however, are similar to those of compound Spitz nevi. A, Under low magnification, of note is an intradermal lesion composed of large blocks of spindle-shaped nevus cells. B and C, Under higher magnification, spindle cells can be observed without atypia and limited cytoplasmatic melanin pigment, as well as presence of Kamino bodies.
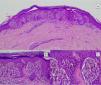
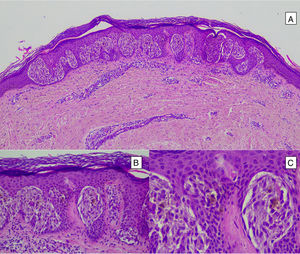
Atypical Spitz tumors do not exhibit all the typical histopathological features of Spitz nevi but they also do not meet the criteria for melanoma. Atypical Spitz tumors show at least 1 of the following features: asymmetry, poor lateral delimitation, greater extension downwards, lack of maturation in the dermis, ulceration, absence of Kamino bodies, presence of mitosis in the dermis (generally more than 2-6 mitosis/mm2), and abundant isolated melanocytes in the superficial dermis instead of nests3,47,48 (Fig. 11).
The presence of a high number of mitosis at depth, asymmetry, high-grade cytological atypia, and ulceration are considered histopathological criteria that correlate with a higher risk of metastasis.47
Pigmented Spindle-Cell Nevus or Reed NevusPigmented spindle-cell nevus is a small, well-defined, melanocytic lesion consisting of a symmetric proliferation of spindle cells with intense pigmentation. The cells are present in rounded nests in a vertical arrangement or a horizontal one at the dermal-epidermal junction. The cells are usually smaller than the spindle cells of most conventional Spitz nevi (Fig. 12). Although mitotic activity may be present, atypical mitosis figures are not usually seen. If they are, we can rule out spindle-cell melanoma, which is extremely uncommon in childhood.44
Desmoplastic Spitz NevusDesmoplastic Spitz nevus is an intradermal proliferation of spindle-cell melanocytes surrounded by abundant thickened collagen strands that form nests at the surface of the skin. The cells are more widely dispersed at the periphery. Mitosis can be found in the superficial parts of the lesion but not in deeper layers. Differential diagnosis should include desmoplastic melanoma.44,49
Combined Spitz NevusCombined Spitz nevus is characterized by the presence of 2 different subtypes of nevi: Spitz nevus and another type of nevus, such as acquired melanocytic nevus or blue nevus.44
Recurrent or Persistent Spitz NevusRecurrence of Spitz nevus after partial or complete excision can resemble melanoma; histological criteria for classic Spitz nevus overlap in terms of presence of asymmetry and pagetoid spread over scarred areas. Diagnosis can be difficult, particularly when multifocal recurrence occurs. It can be useful to reassess the initial biopsy material.50,51
Halo Spitz NevusThe architecture of Spitz nevus is observed with conspicuous inflammatory lymphocytic infiltrate. Clinically, the halo is manifest as erythema, an increase in size, or pruritus at the site of the Spitz nevus; the lesion is usually excised before it reveals the depigmentation halo.44
Pagetoid Spitz NevusPagetoid Spitz nevus is characterized by abundant isolated melanocytes with intradermal pagetoid spread; it can be difficult to distinguish this lesion from in situ melanoma.52,53
Tubular Spitz NevusTubular Spitz nevus is a rare variant of Spitz nevus with a tubular appearance. Intradermal nodules are arranged around an optically empty center. These nodules are composed of the epithelioid cells of the nevus. The lesion can be associated with an intense inflammatory infiltrate.14,44,54
Several theories have been put forward to explain the tubular appearance. Initially the tubular form was considered the product of apoptosis of the central cells of the nevus.54 Subsequently, it was proposed to be an artefact of retraction after fixing in formaldehyde rather than a true variant of Spitz nevus.55
Balloon Cell Spitz NevusBalloon cell Spitz nevi are characterized by the presence of large cells with vacuolated cytoplasm and small basophil nuclei, called balloon cells, in addition to conventional Spitz nevus cells. Balloon cells account for at least 50% of the cells in the nevus.56
Myxoid or Hyalinizing Spitz NevusMyxoid or hyalinizing Spitz nevi occur when myxoid or hyalinizing changes occur in a Spitz nevus.57,58
Plexiform Spitz NevusPlexiform Spitz nevi exhibit the architecture of Spitz nevus with melanocytes that form nests with a conspicuous fascicular dermal growth. Some authors have considered them as a type of atypical Spitz tumor.59
Other VariantsOther variants include polypoid Spitz nevus and verrucous Spitz nevus, both of which are characterized by a markedly exophytic growth pattern.60 Angiomatoid Spitz nevus, originally considered a variant of desmoplastic Spitz nevus, is a classic Spitz nevus with notable vascular proliferation.44,61
Spitzoid MelanomaAs mentioned earlier, spitzoid melanomas can closely resemble Spitz nevi, with nests of epithelioid or spindle-shaped melanocytes associated with a larger number of atypical features than atypical Spitz tumors, such as poor delimitation, asymmetry, extensive pagetoid spread, dermal growth with maturation with depth and no or limited atypical mitoses, as well as high-grade cellular atypia (Table 4).28,29
ImmunohistochemistryImmunohistochemical markers are used as a complementary tool in cases when histopathological diagnosis of Spitz nevus is in doubt, with the aim of better differentiating between Spitz nevus on the one hand and atypical Spitz tumor and melanoma on the other (Table 5). Although immunohistochemistry is useful for identifying malignant characteristics, interpretation can be subjective according to the pathologist.
- •
MIB1: This is a monoclonal antibody that reacts with the protein Ki-67, a nuclear protein implicated in regulation of the cell cycle. It is a marker of proliferation and is more strongly expressed in nuclei of melanomas and atypical Spitz tumors compared with Spitz nevi. Expression decreases progressively from malignant lesions to benign ones, with expression in 37% of melanomas, 10% of atypical Spitz tumors, 5% of Spitz nevi, and 0.5% of conventional nevi.62,63
- •
HMB45: This is a marker of maturation with depth. It is expressed in superficial areas with a decreasing gradient towards the base of the lesion in Spitz nevi while it is destructured with loss of gradient (more uniform distribution, persisting in the dermis) in melanoma. The pattern of expression in atypical Spitz tumors has not been established and a negative result does not rule out diagnosis of melanoma.64
- •
p53: This protein is expressed more weakly in Spitz nevi than in melanoma, although the expression pattern is not constant.65
- •
p21 and cyclin D1: These proteins are overexpressed in Spitz nevi while their expression is decreased in nonspitzoid melanoma.66
- •
p16: This protein regulates the cell cycle, coding for the CDKN2A gene, located on chromosome 9p. It shows more intense reactivity in the dermis in Spitz nevi than in melanoma. It stains intensely in desmoplastic Spitz nevi, unlike desmoplastic melanoma, where it is usually absent.67–69 Three patterns have been identified in spitzoid melanocytic tumors:
- ◦
Homogeneous expression: this pattern is usually observed in Spitz nevi and represents loss of heterozygosity at chromosome 9p or an intact chromosome 9p.
- ◦
Complete loss of expression: this is common in melanoma and corresponds to loss of homozygosity at chromosome 9p, a point mutation in CDKN2A, or methylation of the CDKN2A promotor.
- ◦
Heterozygous loss of expression: this can be observed in compound tumors with mixed clones. It is doubtful whether this is of use for differentiating between atypical Spitz tumors and melanoma, as loss in atypical Spitz tumors can be very variable; 67% of lesions with loss of heterozygosity at 9p21 show expression of p16, whereas those with loss of homozygosity do not express it.70,71
- ◦
- •
E-cadherin: This is an antigen that is diffusely expressed in Spitz nevi and other benign lesions.
- •
CD99: This is a transmembrane glycoprotein more often expressed in melanoma (56%) than in Spitz nevus (5%). In melanoma, staining can be diffuse, unlike in Spitz nevus where the pattern is generally focal when expressed.72
- •
MART1: This is a melanocytic differentiation antigen that is expressed both in Spitz nevus and in melanoma.73
- •
S100: Weak staining in Spitz nevi compared with melanoma.74
- •
S100A6: Staining is more intense and diffuse in Spitz nevus, with a patchy pattern or negativity in Reed nevus, whereas the pattern is weaker and uneven in melanoma.75
Patterns of Expression of Immunohistochemical Markers Used for Differential Diagnosis of Spitzoid Tumors.
| Antigen | Spitz Nevus | Melanoma |
|---|---|---|
| Ki-67 | Limited expression (5%), junction and papillary dermis | Extensively expressed (37%), diffuse |
| HMB45 | Superficial expression, less intense with depth | Loss of gradient, expression maintained with depth |
| p16 | Intense expression | Expression is often lost |
| p53 | Low expression | High expression |
| E-cadherin | Diffuse expression (also in other benign lesions) | Expression is lost |
| S100 | Weak expression | Intense expression |
| S100A6 | Intense and diffuse expression | Weak and uneven expression |
| Cyclin D1 | Intense expression | Low expression in nonspitzoid melanoma |
| p21 | Intense expression | Low expression in nonspitzoid melanoma |
| CD99 | Focal expression | Diffuse expression |
The authors declare that they have no conflicts of interest.
Please cite this article as: Sainz-Gaspar L, Sánchez-Bernal J, Noguera-Morel L, Hernández-Martín A, Colmenero I, Torrelo A. Nevo de Spitz y otros tumores spitzoides en la infancia. Parte 1: aspectos clínicos, histológicos e inmunohistoquímicos. Actas Dermosifiliogr. 2020;111:7–19.