Daylight photodynamic therapy (PDT) is a new type of PDT that is as effective as conventional PDT in mild and moderate actinic keratosis but with fewer adverse effects, resulting in greater efficiency. The climatic conditions in the Iberian Peninsula require an appropriately adapted consensus protocol.
ObjectiveWe describe a protocol for the treatment of grade I and II actinic keratosis with daylight-mediated PDT and methyl aminolevulinate (MAL) adapted to the epidemiological and clinical characteristics of Spanish and Portuguese patients and the climatic conditions of both countries.
MethodsTwelve dermatologists from different parts of Spain and Portugal with experience in the treatment of actinic keratosis with PDT convened to draft a consensus statement for daylight-mediated PDT with MAL in these countries. Based on a literature review and their own clinical experience, the group developed a recommended protocol.
ResultsAccording to the recommendations adopted, patients with multiple mild and moderate lesions, particularly those at risk of developing cancer, are candidates for this type of therapy. Daylight PDT can be administered throughout the year, although it is not indicated at temperatures below 10°C or at excessively high temperatures. Likewise, therapy should not be administered when it is raining, snowing, or foggy. The procedure is simple, requiring application of a sunscreen with a protection factor of at least 30 based exclusively on organic filters, appropriate preparation of the lesions, application of MAL without occlusion, and activation in daylight for 2hours.
ConclusionThis consensus statement represents a practical and detailed guideline to achieve maximum effectiveness of daylight PDT with MAL in Spain and Portugal with minimal adverse effects.
La terapia fotodinámica con luz de día (TFDLD) es una nueva modalidad de terapia fotodinámica (TFD) que, manteniendo la misma eficacia en queratosis actínicas (QA) grado i y ii que la técnica convencional, disminuye sus efectos adversos y la hace más eficiente. Los condicionantes meteorológicos propios de la España y Portugal hacen necesario el establecimiento de un protocolo adecuado y consensuado por expertos adaptado a los mismos.
ObjetivoEstablecer un protocolo para la TFDLD con metil-aminolevulinato (MAL) para el tratamiento de las QA grado i y ii adecuado y consensuado a las características epidemiológicas, meteorológicas y clínicas que se dan en España y Portugal.
MétodoDoce dermatólogos de diferentes áreas geográficas de ambos países, con experiencia en el tratamiento de las QA con TFD, se reunieron para elaborar un documento de consenso para la realización de TFDLD con MAL. De la revisión de la bibliografía y de su experiencia se elaboró el procedimiento recomendado para su realización.
ResultadosLas recomendaciones adoptadas establecen que los pacientes con QA grado i y ii múltiples, especialmente en el contexto de campo de cancerización, son los candidatos a realizar este tratamiento. La TFDLD se puede realizar durante todo el año, siendo limitaciones las temperaturas menores de 10°C o las excesivamente elevadas, así como los días de lluvia, nieve o niebla. El procedimiento es sencillo y requiere la aplicación de un fotoprotector FPS>30 que solo contenga filtros orgánicos, la preparación adecuada de las lesiones, la aplicación del MAL sin oclusión y su activación con la luz del día durante 2h.
ConclusiónEste documento de consenso supone una guía práctica y detallada para la realización de la TFDLD con MAL en España y Portugal destinada a la consecución de la máxima efectividad con mínimos efectos adversos.
Actinic keratoses (AKs) are very common skin lesions that appear in areas that have received long-term exposure to UV radiation.1 They are considered precancerous by some authors and in situ squamous cell carcinomas by others. The prevalence in adults over the age of 45 years in Spain is estimated to be 28.6% (95% CI, 27.2%–30.1%).2 Treatment is considered imperative because a certain percentage of these lesions—ranging from 0.60% in the first year to 2.57% over 4 years—will progress to invasive squamous cell carcinoma.3
As a result of progress in clinical practice in recent years, supported by an abundance of published data, photodynamic therapy (PDT) has become one of the established treatments for AK (evidence level, A.1).4 In addition to the demonstrated efficacy of this therapy, it has been associated with few adverse effects and good cosmetic results.5–8 One of the main disadvantages of conventional PDT, however, is intense pain during exposure to the light source. Pain has even occasionally been associated with raised blood pressure, and analgesia, local anesthesia, nerve blocks, or sedation have been necessary for some patients.9–11 Another issue is the required level of preparation: a PDT-specific lamp must be used and the staff will need to receive special training. In conventional PDT, the photosensitizer—either aminolevulinic acid or methyl aminolevulinate (MAL)—must be applied and kept covered (occlusion) for 3hours to incubate before the area is exposed to an appropriate light source that delivers between 37 and 100J/cm2. Therefore, daylight PDT is simpler than the conventional method and causes fewer adverse effects. In particular, there is less pain.
Daylight PDT with MAL (Metvix; Galderma, Paris, France) has proven to be as effective as the conventional modality but it is better tolerated and more efficient.12,13 Natural daylight is the source of irradiation. The photodynamic effect is continuous, since the active metabolite of MAL, protoporphyrin IX (PpIX), is activated by daylight exposure as it is produced. Since PpIX does not accumulate in the skin, the procedure is less painful.14
The first European consensus paper on the use of daylight PDT, published in 2011,15 summarized the results of 4 phase-3 randomized clinical trials supporting the modality's efficacy in treating AK. The reported complete response rates at 3 months are similar (79% for daylight PDT vs 71% for the conventional modality). Pain is reported to be nearly absent (scores of 2 vs 6.7, respectively, on a scale of 0 to 10), and both patients and clinicians consider the daylight modality to be more convenient.13,14,16,17
However, since that paper appeared several other studies and 2 clinical trials18,19 of interest have been published. The most important one to date is an Australian multicenter randomized noninferiority trial that showed that daylight PDT is similarly effective to conventional PDT for treating mainly mild AKs; the 3-month complete response rates were 89% vs 93% for the 2 modalities, respectively (95% CI, –6.8% to –0.3%).18 The natural light source was also better tolerated during exposure, and the response was maintained over the 6-months of follow-up in 96% of the lesions.18
A similar multicenter trial conducted in 5 countries in northern and southern Europe also found no significant differences in the response rates of mild to moderate AK treated with conventional (75%) or daylight-PDT (70%).19 Moreover, response in that study was not influenced by weather conditions (sunny, cloudy, or partly cloudy) on the day of treatment. Pain scores were significantly lower with the new PDT modality than with the conventional method (0.7 vs 4.4, P<.001). Other trials in different locations (e.g., Brazil, Italy, Spain), or in which aminolevulinic acid was the photosensitizer, have reported similar results.20–25 A finding that is consistent across these trials is the lower rate of adverse effects, particularly pain, with daylight PDT.
In the light of evidence from these new trials, and given the considerable advantages of this new approach for both the patient and the dermatologist, a new European consensus paper was recently published.26 However, as daylight PDT is subject to local conditions of sunlight and climate as well as seasonal variation, consensus papers must be drafted for particular geographic areas. In consideration of the significant variations of climate over the course of a year in Spain and Portugal, dermatologists expert in PDT from these 2 countries met to agree on how to use this modality to treat AK.
This article summarizes the main recommendations the experts put forth at the meeting. They are based on a review of the available literature and the experience of the experts regarding patient selection and the procedures to follow.
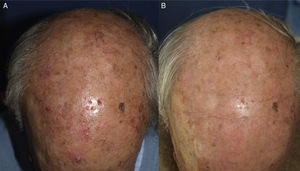
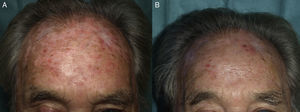
Procedures for Daylight PDTPatient SelectionOn the basis of available evidence, and consistent with the European guidelines,26 daylight PDT should be used to treat multiple grade 1 or 2 AK lesions, especially when a wide area of exposed skin is involved. Patients who cannot tolerate conventional PDT are prime candidates. Daylight PDT is particularly advantageous for field cancerization, as both visible and subclinical lesions (Fig. 1) will be treated. Patients with as-yet untreated AK as well as those who have been treated by any other available modality (Figure 2), including conventional PDT, are also candidates. Treatments can be repeated, although the most appropriate interval between them has not yet been established.
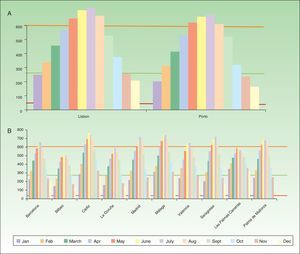
Based on the results of the 2 main clinical trials described above,18,19 we conclude that 2hours of exposure to sunlight can be recommended to ensure synthesis of a sufficient amount of PpIX and activation of the photodynamic effect. Given that sunlight varies in intensity over the course of a year, the daily means for several cities in Spain and Portugal for the hours between 9a.m. and 6p.m. (Fig. 3A, 3B) were calculated using specific software for analyzing climate data (Meteonomr; Meteotest, Bern, Switzerland). The minimum intensity required for daylight PDT has been established as 130W/m2. Therefore, a comparison of the minimums, means, and maximums reported in the European clinical trial19 shows that the levels of sunlight received throughout the year in all the Spanish and Portuguese cities exceed the requirements. Thus, the geographic situations of Spain and Portugal allow the solar irradiation threshold for PDT to be reached throughout the two territories at any time of year.
Daily measurements of natural light intensity between 9a.m. to 6p.m. (Watts/m2) over the course of a year in A) Portuguese and B) Spanish cities. Shown are daily means by month (Meteonorm data 1986–2005). The colored horizontal lines show the intensity levels reported in a European trail of daylight-mediated PDT.25 Red refers to the lowest intensity, 44W/m2; orange, the highest, 601W/m2; and green, the mean, 267W/m2.
The patient should be exposed to sunlight between 10a.m. and 6p.m., the time frame when light doses have been measured in previous studies. On short midwinter days, however, exposure should not begin any later than 3p.m. In summer, on the other hand, exposure can continue up to 7p.m.
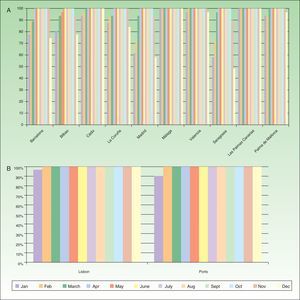
Recommended Temperatures for Daylight PDTLower PpIX production has been linked to low temperatures in preclinical trials. It is also difficult for patients to spend time outdoors at low temperatures, so the recommended minimum is 10°C in the interest of patient comfort.26 Most cities in Spain and Portugal record temperatures that meet this requirement throughout the year. Some cities in northern Spain, however, (e.g., Madrid and Saragossa) report lower temperatures on about 40% of the days in December and January (Fig. 4A, 4B). Extremely high summer temperatures should also be taken into consideration, as they can affect this PDT modality in 2 ways: sweat can decrease the efficacy of treatment, and elderly patients may suffer heat stroke. In hot weather, it is possible for patients to receive PDT in an area that provides light shade.
In fact, PDT can be provided in any weather conditions except rain.
Recommended ProtocolTable 1 gives an overview of the steps in this protocol, which uses MAL as the photosensitizer.
Protocol for Daylight PDT with Methyl Aminolevulinate.
| Step | Procedures | Notes | Illustrations |
|---|---|---|---|
| Sunscreen | The patient applies a sunscreen (SPF 30–50+) on all skin surfaces that will be exposed either before coming to the treatment session or about 15min before application of MAL. | Do not use a sunscreen with physical filters (i.e., use products without titanium dioxide or zinc oxide). | |
| Skin preparation | Hyperkeratotic scaling should be eliminated from the area to be treated. | Use a curette, abrasive paper, a product with urea or salicylic acid, laser, or microneedling. | |
| MAL application | Apply a thin layer of MAL on the AKs to be treated. | No occlusion necessary. Use 1g to treat a complete scalp or face. | |
| Daylight exposure | The patient must go outside within 30min after application of MAL.Outdoor daylight exposure for 2h under direct sunlight or light shade.Start no later than 3p.m. in winter.In summer, exposure can continue up to 7p.m. (to avoid the hottest hours of the day). | Efficacy is the same on sunny and cloudy days.The temperature must be over 10°C.Avoid shade from buildings.The patient must go outside within 30min so that PpIX does not build up in the skin and cause pain on exposure. | |
| MAL removal and precautionary measures | Wash off the MAL after 2h of exposure.Cover the treated area for 24h with a physical dressing or, if that is not feasible, use a sunscreen (SPF 50+). | The MAL can be removed by clinic staff or by the patient.The sunscreen should contain physical filters.Use a moisturizer for a week to alleviate the problem of crust formation. | |
| Follow-up | Evaluate the treated AKs 3 mo later. | Re-treat in another session if necessary. |
Abbreviations: MAL: methyl aminolevulinate; PpIX: protoporphyrin IX; SPF: sun protection factor.
A sunscreen is recommended to prevent excessive UV irradiation and sunburn during exposure. The product should contain only organic or chemical filters: inorganic filters not only block UV radiation (the desired effect) but they also filter out the visible light that is necessary to stimulate PpIX and initiate the photodynamic response.
The sun protection factors (SPFs) of products used in trials have ranged from 15 to 50, giving similar results without adverse effects. Therefore, the usually recommended SPF is 30 or higher. To ensure adequate absorption of the sunscreen, so that it is effective, the patient should apply it to all exposed areas before arriving for treatment. If the patient has not done so, it can be applied in the office and followed by a wait of about 15minutes.
Lesion PreparationBefore applying the photosensitizer to the targeted area, remove any scaling skin using one of several available approaches: abrasive paper, keratolytic creams containing urea or salicylic acid, microneedling, or even laser ablation.20,27 Most PDT experts choose curettage, the method used in both the European and Australian trials.18,19
Photosensitizer ApplicationApply a thin layer of MAL cream on the area to be treated—the lesion or the field. A general recommendation is to use 1g to treat the entire scalp or face. Occlusion is not necessary, and the patient should be seated outside within the next half hour. If the patient lingers inside, PpIX can begin to accumulate in the skin, increasing the likelihood of pain on exposure to daylight.
Daylight ExposureOutdoor exposure should last 2hours to ensure adequate synthesis of PpIX in the lesions and activation of a satisfactory photodynamic response. Although exposure is generally to direct sunlight, light shade can be used on hot days in the interest of comfort. Areas of deep shade created by buildings are inappropriate.
AftercareAfter 2hours of exposure, remove any MAL cream from the skin with water or normal saline. The treated area should then be covered for at least 24hours. However, if occlusion is not feasible, cover the treated area with a sunscreen (SPF 50+) that contains inorganic (i.e., physical) particles. In fact, some experts choose only this option and have not observed that adverse effects are more frequent or more intense. The patient can be advised to use a moisturizer over the next week to alleviate the discomfort of crust formation.
Follow-upPatients should be followed according to the same schedule used after conventional PDT. A follow-up visit usually takes place at 3 months unless the dermatologist feels an earlier one is advisable.
ConclusionsThis consensus paper provides guidelines for treating AK with daylight PDT in Spain and Portugal. The new PDT modality offers important benefits over the conventional method for treating AK lesions or field cancerization. Patients experience fewer adverse effects, especially less pain. Staff save time, as anesthesia is unnecessary and illumination does not need to be monitored closely. Thus, the treatment center saves on the allocation resources (staff, facilities, and the purchase of specific equipment). Daylight PDT should not be considered a substitute for the conventional technique, however, but rather an alternative that is particularly appropriate for patients who cannot tolerate conventional PDT or who require treatment of extensive areas of skin. Conventional PDT continues to be the only technique indicated for Bowen disease and both superficial and nodular basal cell carcinoma (depth, <2mm).4
In conclusion, daylight PDT is a safe, effective alternative for treating mild and moderate AK. It is more comfortable for patients and, hence, better tolerated than conventional PDT, and it is easier for health care staff to apply.
FundingThe pharmaceutical company Galderma promoted this consensus statement without interfering with the decisions of the expert group.
Conflicts of InterestDr Yolanda Gilaberte has spoken at events or participated in clinical trials sponsored by Galderma S.A., Leo Pharma, Almirall, and Novartis. Dr Carlos Serra has spoken at events sponsored by the following pharmaceutical companies: IFC, MEDA, and Leo Pharma. He has participated in clinical trials sponsored by Galderma, S.A. and Leo Pharma. Dr Carlos Guillén has spoken at events sponsored by IFC, MEDA, and Leo Pharma. He has also participated in clinical trials sponsored by Galderma, S.A. and Leo Pharma. Dr Bibiana García has spoken at events or participated in trials sponsored by Galderma S.A. Dr Antonio Harto has received fees for lectures and training seminars sponsored by Galderma S.A. Dr Pedro Redondo has spoken at events sponsored by Galderma S.A.; he was principal investigator for a trial funded by the same company. The expenses of Dr Lidia Pérez Pérez while attending Spanish and international conferences on PDT have been paid by Galderma S.A. Drs Miguel Aguilar, Luis Miguel Valladares, and Manuel Almagro declare that they have no conflicts of interest.
Please cite this article as: Gilaberte Y, Aguilar M, Almagro M, Correia O, Guillén C, Harto A, et al. Documento de consenso hispano-portugués para el uso de la terapia fotodinámica con metil aminolevulinato y luz de día en el tratamiento de las queratosis actínicas. Actas Dermosifiliogr. 2015;106:623–631.