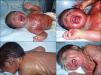
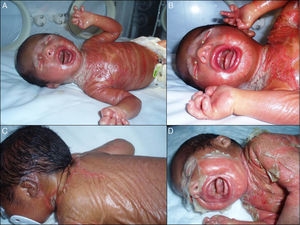
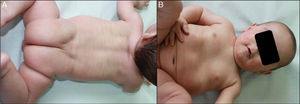
We present the case of a newborn infant born preterm at 36 weeks, with no family history of interest, diagnosed in utero with eclabium and ectropion, with a suspected diagnosis of harlequin fetus. At birth, the infant presented a membrane that covered practically the whole body surface like a suit of armor (Fig. 1A), in addition to ectropion, eclabium, clawed-hand deformity (Fig. 1B), and retraction of the auricles of both ears (Fig. 1C). During the first days after delivery, the membrane fissured and subsequently peeled away in large sheets (Fig. 1D), leaving the underlying skin erythematous and exudative; the membrane had fallen completely by the second week of life. The clinical course was very good over the following months, and at 1 year the skin had a practically normal appearance (Fig. 2A), showing only mild erythema and peeling on both cheeks (Fig. 2B) and minimal hyperkeratosis on the elbows and knees. A diagnosis of self-healing collodion baby (CB) was made. Genetic analysis showed the child to be a homozygous carrier of a mutation in the ALOX12B gene that produced loss of an amino acid, glutamine, at position 136 of exon 3; the parents were healthy heterozygous carriers of this mutation. At the time of writing this article, the patient was 2 years of age and had no lesions except those commented above.
A, Shiny membrane covering practically the whole body surface like a suit of armor. B, Ectropion, eclabium, and clawed-hand deformity. C, Retraction of the auricle of the left ear and view of the membrane over the back with a central fissure in the dorsal region. D, Peeling of the whole membrane in large sheets.
CB presents at birth with a tight, shiny, transparent, armor-like membrane that covers the whole body surface and looks like cellophane wrapping.1,2 This can give rise to ectropion, eclabium, pseudocontractures, absence of eyebrows, sparse hair, and hypoplasia of the nasal and auricular cartilage.1,2 The membrane is inelastic, so the child's breathing and movements after birth provoke fissuring, and it then peels away in large sheets and is completely lost by 2 to 4 weeks of life.3 CB is a rare condition, with an incidence between 1 in 50000 to 1 in 100000 births. It is the initial clinical manifestation of a number of genetic diseases, the majority of which belong to the group of autosomal recessive congenital ichthyoses (ARCI).4 CB can develop into very diverse phenotypes, from skin with a normal appearance to intense ichthyosis; the majority of patients are diagnosed with lamellar ichthyosis or congenital ichthyosiform erythroderma.1,3 Self-resolving CB is a minor form of ARCI.4 Between 10% and 24% of cases of CB are self-resolving; these are cases that show spontaneous resolution of the condition and in adult life present a normal skin or discreet signs of ichthyosis.1,5 Regarding the epidemiology of ARCI, Hernández-Martín et al., in Spain, published a study in which the estimated prevalence of ARCI was of approximately 16 cases per million population, with a prevalence of self-resolving CB of 4.2% in the overall ARCI population.6
Self-resolving CB has been associated with mutations in genes TGM1, ALOXE3, and ALOX12B.1,3–5,7 Our patient was diagnosed as a homozygous carrier of a mutation in gene ALOX12B that has not previously been described in the literature. The ALOX12B gene was first identified in 2002. It is formed of 15 exons and codes for the epidermal lipoxygenases eLOX-3 and 12R-LOX.8 Its predominant expression in the suprabasal layers of the epidermis supports its role in the advanced phases of epidermal differentiation and its participation in processing lamellar bodies. In addition, it acts in the hepoxilin pathway and is therefore thought possibly to participate in the formation of the intercellular lipids of the corneal layer or to act in signaling to promote keratinocyte differentiation.8
More than 30 mutations of the ALOX12B gene have been described since its identification and, together with gene ALOXE3, it is considered responsible for 14% to 17% of ARCIs and for 72.2% of cases of self-resolving CB.8 The specific mechanism that leads to the changes in skin permeability in patients with alterations of gene ALOX12B, and the reason for the appearance of lesions in the neonatal period in self-resolving CB, are not fully understood.7 It has been postulated that these mutations of ALOX12B reduce enzyme activity in utero, but not after birth.8 Hydrostatic pressure is high in the uterus, and the chelation of water molecules deforms the mutated enzyme into an inactive conformation. After birth, with a lower hydrostatic pressure, the enzyme returns to its active form and increases its activity to levels sufficient to maintain a normal or minimally altered phenotype.8,9
CB usually causes premature delivery, with increased perinatal morbidity and mortality. Important complications include increased transepidermal water loss (up to 7 times the loss from healthy skin), temperature instability, hypothermia, hypernatremic dehydration, feeding difficulties, hypohidrosis, cutaneous and systemic infections, ectropion, keratitis, and obstruction of the external auditory meatus.1,2,5,10 Furthermore, the membrane can produce mechanical compression that may lead to distal limb ischemia.2
The management of CB must be undertaken in a neonatal intensive care unit. Humidity in the incubator should be at least 60%, with temperature control, monitoring of electrolytes, energy supplementation, and insertion of a nasogastric tube if feeding is compromised. Although prophylactic antibiotics are not recommended, very close monitoring must be performed and antibiotic therapy initiated early if signs of infection develop.2 The use of emollients reduces transepidermal water loss and its related complications, though health staff must perform adequate hand washing and hygiene prior to applying the agents in order to reduce the risk of infection, as this has been related to the application of emollients in some reports.2
Mortality in CB is currently around 5%; in 1960 it was estimated to be around 50%.2,10 It is believed that the marked improvement in prognosis is due to the advances in neonatal care.2
Reporting this new case of CB is important because of its progression to self-resolving CB, a very rare entity. Self-resolving CB is a challenge not because of diagnostic problems, but rather because of the difficulty in establishing a long-term prognosis, as it is impossible to say whether a case will be a true self-resolving CB or whether it will, in contrast, develop into a very severe disease. Furthermore, the genetic analysis performed on our patient detected a mutation in the ALOX12B gene that had not previously been described in the literature as a mutation confirmed to cause self-resolving CB.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Muruzábal RS, Irurzun AL, Bayona IY, Arroyo MAR. Bebé colodión autorresolutivo: nueva mutación en el gen ALOX12B. Actas Dermosifiliogr. 2016;107:433–435.