Secondary amyloid deposition is occasionally associated with skin tumors and inflammatory skin disorders, but it is rarely seen in discoid lupus erythematosus (DLE). We describe a case of secondary amyloid deposition in the lesional skin of DLE.
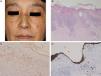
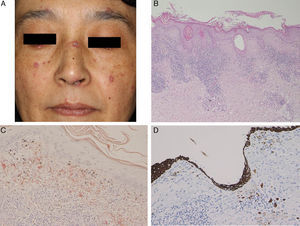
A 47-year-old woman presented with keratotic erythema on the nose 3 years ago. The erythema had been present for 1 year and the histological features were compatible with DLE. Three years later, she revisited our hospital because the scaly erythematous patches had gradually increased in number. Physical examination revealed several reddish, well-circumscribed plaques with slightly elevated borders on the forehead, nose, cheeks, lip, and ear (Fig. 1A). Laboratory data showed leukocytes within the normal range (8100mm−3 with 32% neutrophils), normal liver and renal function, elevated immunoglobulin (Ig) G (2172mg/dL), and positive titers of antinuclear antibody (1:1280), anti SS-A antibody (37.0U/mL; normal, <7.0U/mL), and anticentromere antibody (index of 34.4; normal, <10). Serum complement levels and antibodies against SS-B, ds-DNA, and Sm were all within normal limits. A second biopsy taken from a lesion on the dorsum of the nose showed parakeratosis, follicular plugging, liquefaction of the basal layer, and focal cellular infiltration in the dermis (Fig. 1B). There was eosinophilic material in the upper dermis, which was positive with Congo red and Dylon staining (Fig. 1C) and anticytokeratin antibody 34βE12 staining (Fig. 1D). The direct immunofluorescence study revealed IgM deposits at the dermal-epidermal junction, but no amyloid deposits were observed in another DLE specimen taken from the back of the ear. A detailed eye exam revealed decreased lacrimation, but saliva production measured by the Saxon test was normal.
(A) Multiple keratotic plaques scattered on the face. (B) Biopsy showed parakeratosis with focal hyperkeratosis, follicular plugging, liquefaction changes to the basal layers of the epidermis, and cellular infiltrates in the upper dermis (hematoxylin–eosin stain, original magnification 100×). (C) Dylon staining revealed amyloid deposition in the upper dermis (original magnification 200×). (D) The upper dermis was also positive for keratin (original magnification 200×).
Few papers have reported on secondary amyloid deposition in DLE, but it has been suggested that this phenomenon might be underreported.1 Powell et al.1 reported 3 cases of DLE on the head and neck with amyloid deposition. They also retrospectively examined 18 cases of DLE, and detected amyloid deposition in 1 case. In a Japanese series, Khan et al.2 detected amyloid deposition in all 4 cases of hypertrophic DLE analyzed but in just 1 of 12 cases of nonhypertrophic DLE. They speculated that cutaneous amyloid material might have been deposited secondary to abnormalities in the basement membrane zone following repeated sunlight exposure for long periods. Our case was not hypertrophic-type DLE, but amyloid deposition was detected on the nose, which had an evident keratotic appearance. The fact that amyloid deposits were not detected in the DLE lesion on the back of the ear suggests that UV radiation may play a role in inducing basement membrane impairment, resulting in secondary amyloid deposition. The amyloid material also stained positively for 34βE12, suggesting that the origin of the amyloid was degenerating epidermal keratinocytes, which may be precursors of cutaneous amyloid. The presence of lamina densa-like substances or disturbed keratinization in the basal layers might also be involved in amyloid production.3 Our patient also developed Sjögren syndrome, although she did not show any symptoms of dry mouth. Because systemic sclerosis was ruled out, the presence of serum anticentromere antibody was considered to be associated with Sjögren syndrome, although coexistence of this condition with DLE is rare.4 Anticentromere antibody has been detected in 10% of Japanese patients with primary Sjögren syndrome5; these patients had lower mononuclear cell infiltration in the minor salivary glands, possibly explaining the lower frequency of dry mouth in this subgroup of patients. This hallmark symptom of Sjögren syndrome was also absent in our patient. In conclusion, there have been few reports on secondary amyloid deposition in DLE, but there may be more cases, especially in hyperkeratotic lesions in sun-exposed areas.