Metastasis is the main cause of death from melanoma. Chemokines are low molecular weight chemotactic cytokines that facilitate cellular migration. Thus, cells that express receptors for a given chemokine are attracted to the site of its production. As certain chemokines are found in abundance in organs that are common targets of metastasis and receptors for these chemokines are expressed by tumor cells, it was hypothesized that chemokine gradients might selectively facilitate metastasis to these organs. A later finding that these chemokines were produced by tumor cells, with evidence of autocrine effects, obliged the modification of that hypothesis. Many chemokines are also known to have opposing effects according to the type of cell they are acting on (tumor, inflammatory/immune, or endothelial cells), their functional status, or interactions with other molecules. The expression of chemokines and their receptors by melanoma cells enhances tumor progression by altering their microenvironment, stimulating angiogenesis, and inhibiting the immune response.
Las metástasis son la principal causa de mortalidad en el melanoma. Las quimiocinas son citocinas quimiotácticas de bajo peso molecular que permiten la migración celular, atrayendo a células que expresan sus receptores. Como algunas quimiocinas son abundantes en órganos que son asiento común de metastasis, y sus receptores son expresados por las células tumorales, se planteó la hipótesis de que un gradiente de estas quimiocinas facilitaría selectivamente las metástasis a esos órganos. El hallazgo posterior de la producción de estas quimiocinas por las propias células tumorales, y su efecto autocrino, obliga a modular esta hipótesis. Además, muchas quimiocinas tienen efectos contrapuestos dependiendo del tipo celular sobre el que actúan (tumoral, inflamatorio/ inmunitario, endotelial), su estado funcional y otras interacciones moleculares. En el melanoma las células tumorales adquieren ventajas de la expresión de quimiocinas y receptores alterando su microambiente, estimulando la angiogénesis y evadiendo la respuesta inmune, facilitando así la progresión tumoral.
Melanoma has increased in frequency more than any other cancer in recent years, and in affected patients the development of metastasis is the strongest predictor of mortality, given the absence of clearly effective treatments for metastatic disease.1 The expression of certain chemokines and their receptors has been implicated in the molecular mechanisms that favor or inhibit metastatic spread of melanoma.2–5 Understanding the molecular mechanisms underlying tumor progression in melanoma is an important step that will ultimately allow not only prediction but also therapeutic intervention.
The ChemokinesThe chemokines are low-molecular-weight (8-17 kDa) chemotactic cytokines that cause directed cell migration and whose expression is induced by inflammatory cytokines, growth factors, and pathogenic stimuli.3,5 They have been classified into 4 families: CXC, CC, CX3C, and XC chemokines, defined according to the position of the first 2 cysteine residues located in the amino-terminal region. To date, more than 50 chemokines and at least 19 different chemokine receptors have been identified3,5 (Table 1). Although in many cases the same receptor can recognize various different chemokine ligands, 6 of the known chemokine receptors only recognize a single ligand. It is thought that any cell type can express chemokines or their receptors.6 The expression of chemokine receptors is dependent upon cell type, differentiation state, the concentration of chemokines present in the microenvironment, the presence of inflammatory cytokines, and the occurrence of local hypoxia.5 The binding of chemokines to their receptors at the cell surface leads to a complex series of cellular responses, such as activation of different signaling pathways and cytoskeletal reorganization, which favor the formation of pseudopodia and facilitate cell movement. Chemokine receptors act through heterotrimeric G proteins that regulate signaling pathways such as those involving mitogen-associated protein kinases, phospholipase C-β, phosphoinositol-3-kinase, RAS, and Rho-family GTPases.4,7,8 RhoC is thought to play an important role in the occurrence of metastasis.9 Chemokines can also act in synergy with metalloproteinases.10–12
Classification of Chemokines and Their Receptors.
| Family | Receptor | Chemokines |
| CXC | CXCR1 | CXCL6 and 8 |
| CXCR2 | CXCL1, 2, 3, 5, 6, 7, and 8 | |
| CXCR3 | CXCL9, 10 and 11 | |
| CXCR4 | CXCL12 | |
| CXCR5 | CXCL13 | |
| CXCR6 | CXCL16 | |
| CXCR7 | CXCL11 and 12 | |
| CC | CCR1 | CCL3, 4, 5, 7, 14, 15, 16, and 23 |
| CCR2 | CCL2, 7, 8, 12, and 13 | |
| CCR3 | CCL5, 7, 11, 13, 15, 24, 26, and 28 | |
| CCR4 | CCL2, 3, 5, 17, and 22 | |
| CCR5 | CCL3, 4, 5, and 8 | |
| CCR6 | CCL20 | |
| CCR7 | CCL19 and 21 | |
| CCR8 | CCL1, 4, and 17 | |
| CCR9 | CCL25 | |
| CCR10 | CCL27 and 28 | |
| CX3C | CX3CR1 | CX3CL1 |
| XC | XCR1 | XCL1 and 2 |
One of the great enigmas that has faced oncological pathology in the last 2 centuries is the question of how metastasis selectively targets some organs and not others irrespective of the blood flow they receive and, therefore, the quantities of circulating tumor cells that reach them.13 In 1889, after studying more than 900 autopsies, Stephen Paget14 proposed the “seed and soil” hypothesis to explain this phenomenon. According to this theory, metastases do not occur randomly; instead, certain tumor cells (the “seeds”) have a particular affinity for the microenvironment of the affected organ (the “soil”).
The site of distant metastases also has prognostic value in patients with melanoma. Metastases found in the skin, subcutaneous tissue, and lymph nodes are associated with longer survival than visceral metastases. Consequently, the American Joint Committee on Cancer subclassifies metastases into M1a for distant cutaneous, subcutaneous, and lymph-node metastases, M1b for lung metastases, and M1c for metastases to other visceral sites.15
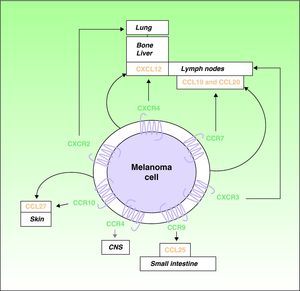
Over the last decade, it has been recognized that chemokines and their receptors play a role in metastatic spread.4,16 Muller et al4 observed overexpression of CXCR4, CCR10, and CCR7 in melanoma cell lines. Since CXCL12, the ligand for the receptor CXCR4, is preferentially detected in the lymph nodes, lungs, liver, and bone marrow, the authors proposed a role for the receptor in the selective spread of metastases to these sites (Fig. 1). In support of this hypothesis, expression of CXCR4 has been detected in subcutaneous and lymph-node metastases of melanoma in humans and in melanoma cell lines, where it facilitates the adhesion and migration of tumor cells through interaction with its ligands.7 Interactions between CXCR4 and CXCL12 are responsible for cell migration during embryogenesis and the mobilization of hematopoietic stem cells,5,17 and they have a recognized role in metastatic spread.4,5 At least 1 subpopulation of tumor cells expresses CXCR4 in many types of cancer.5 Situations such as hypoxia that increase activation of the so-called hypoxia-inducible factor cause an increase in the expression of CXCR4 by tumor cells.18 The only known ligand of CXCR4 is CXCL12, a 68-amino-acid chemokine that has angiogenic properties,19 promotes tumor growth,19,20 and facilitates tissue invasion by increasing the production of various metalloproteinases.11 In the B16 cell line, a well-characterized mouse melanoma cell line with known metastatic potential, tumor cells transfected with CXCR4 are reported to have a 10-fold greater capacity to cause lung metastases.21
Involvement of chemokines and their receptors in selective metastasis in patients with cutaneous melanoma. Black arrows indicate well-characterized interactions between chemokines and their receptors; gray arrows indicate potential associations that remain to be fully characterized; curved arrows indicate the autologous production of chemokines (ligands) by the tumor cells leading to autocrine effects. CNS indicates central nervous system.
The receptor CCR7 also plays an important role in melanoma, and its ligands (CCL19 and CCL21) are expressed in dermal lymph vessels, paracortical areas of the lymph nodes, and high endothelial venules of the lymph nodes.22,23 The interaction of CCR7 with its ligands has been implicated in the development of lymph-node metastases in an experimental mouse model of melanoma23 (Fig. 1). Mechanisms have also been described to control the expression of the ligands of CCR7, particularly CCL21, in lymph nodes where melanoma metastasis has occurred.24,25 As we will see, however, the role of CCL21 and CCR7 in metastasis is complex.
The tendency of melanomas to produce skin metastases is a well-known phenomenon. A role in this process has been proposed for the receptor CCR10, whose ligand CCL27 is predominantly expressed in the basal cells of the epidermis4 (Fig. 1). Patients with hematogenous spread of melanoma often also have gastrointestinal metastases.26 CCR9 participates in the development of metastases in the small intestine, which expresses its ligand CCL2527 (Fig. 1). The receptor CCR4 has also recently been implicated in cerebral metastases of melanoma28 (Fig. 1).
It has also been shown in recent years that tumor cells from various types of cancer, including melanoma, express not only chemokine receptors but also the chemokines themselves. This coordinated expression thus generates circuits or autocrine feedback loops in the tumor cells themselves. Notable examples are CXCL12 and its receptor CXCR4 and CCL21 and its receptor CCR7, which play an essential role in tumor growth and migration, and therefore the aggressiveness of the tumor cells. These autocrine loops stimulate not only the proliferative capacity but also the invasive capacity or metastatic potential of tumor cells.29,30
AngiogenesisAngiogenesis is essential for tumor growth and correlates with the development of metastasis. The role of chemokines in this process is also complex. In general, CXC chemokines containing glu-leu-arg (ELR according to the single-letter code for amino acids) domains—specifically CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8 (all ligands of CXCR2)—function as angiogenic cytokines, although CXCL12, which does not contain ELR domains, also has angiogenic effects.3 CXCL8 is a particularly angiogenic chemokine and its receptors CXCR1 and CXCR2 are expressed on endothelial cells.31 CXCL8 exerts its effect largely by inducing expression of the metalloproteinases MMP2 and MMP9, and also vascular endothelial growth factor, which has an autocrine effect on its receptor in the same endothelial cells.31 In contrast, CXCL4, CXCL9, CXCL10, and CXCL11 are antiangiogenic.3
Immune ResponseChemokines act as mediators in the recruitment of different types of cells in the tumor microenvironment; these include lymphocytes, tumor-associated macrophages (TAM) and neutrophils (TAN), fibroblasts, mesenchymal stem cells, and endothelial cells.3 Two types of TAM and TAN have been identified: type 1, which are antitumoral, and type 2, which are protumoral.32,33 The T-helper 1 (TH1) lymphocyte response, which is greater in regressing melanomas,34,35 has an antitumoral effect, whereas the TH2 response facilitates tumor progression. Nevertheless, the situation is complex. This is highlighted by the chemokines CXCL10 and CXCL4, which both have affinity for the receptor CXCR3 but nevertheless have opposing effects. CXCL10 induces production of the TH1 cytokine interferon-γ by T cells, whereas CXCL4 promotes expression of the TH2 cytokines interleukin 4, 5, and 13.36 CXCL10 appears to act preferentially on CXCR3A, whereas CXCL4 binds a variant of the same receptor, CXCR3B.36 There is increasing evidence that the chemokines present in human tumors contribute more to tumor growth and progression and to immunosuppression than they generally do to an effective antitumor response.37 What is clear is that the interactions between chemokines and their receptors sometimes potentiate and sometimes inhibit the immune response. Thus, the expression of CCL27 is an important inducer of the immune response in squamous cell tumors and its inhibition contributes to an evasion of the immune response and favors tumor progression.38 In fact, expression of CCL27 is lower in melanoma metastases than in primary cutaneous melanomas.39 However, overexpression of CCR10, the receptor for CCL27, in the B16 melanoma cell line, probably stimulated by high levels of CCL27 expression in the epidermis, appears to increase the resistance of these cells to the immune response against the tumor and protects against Fas-mediated apoptosis.40 In fact, overexpression of CCR10 in B16 melanoma cells leads to a notable increase in the occurrence of lymph-node metastases in mice following injection of the transfected cells into the skin.41 Increased expression of CCR10 has also been observed by immunohistochemistry in primary melanomas from patients with positive sentinel lymph node biopsy compared to those in whom biopsy was negative for metastasis.41
Contradictory data have been obtained regarding the influence of CXCL12 on the antitumoral immune response. The production of CXCL12 by tumor cells can reduce the immune response to the tumor by attracting and promoting the survival of dendritic cell precursors (pre-DC2) that express CXCR4 and altering the distribution, immunity, and stimulus of fibrosis of pre-DC1 cells, causing immature dendritic cells not to present tumor antigens.42 However, it has also been reported that the production of CXCL12 by B16F1 melanoma cells that have been genetically modified to produce the cytokine and injected into immunocompetent mice led to rejection in 50% of cases and induced specific memory responses to the tumor.43 Consistent with this observation, secretion of CXCL12 by genetically modified melanoma cells attracts CXCR4-positive cutaneous T lymphocytes in organotypic cultures and facilitates tumor regression.44
One of the most recent and striking advances in relation to the role of chemokines in the immune response relates to CCL21. Under normal circumstances, this cytokine is produced by fibroblastic reticular cells (FRC) in the paracortex of the lymph nodes and attracts both antigen-presenting cells and melanoma cells to that site, since both express CCR7. It has been shown that the tumor cells from different types of cancer, including melanoma, can produce CCL19 and CCL21 under conditions of slow interstitial flow and lead to an autocrine effect referred to as autologous chemotaxis, which potentiates cell migration in the direction of the flow towards the lymph vessels.30 Comparison of the behavior of B16 melanoma cells expressing high and low levels of CCL21 following implantation into syngeneic immunocompetent mice revealed that cells expressing high levels of the chemokine produced larger tumors despite attracting more leukocytes and antigen-presenting cells. This effect, which is not observed in vitro, depends on the host response, since it does not occur in immunodeficient mice. In other words, high endogenous expression of CCL21 in melanoma cells favors immune tolerance and therefore progression of the tumor.45 Tumors expressing high levels of CCL21 have more CD4+, CD25+, FoxP3+ regulatory T cells and express more transforming growth factor β1, which favors phenotypic change of M1 (antitumoral) to M2 (protumoral) macrophages. Lymphoid tissue inducer cells were also present along with a network of FRC cells and some vessels with characteristics of high endothelial venules. However, tumors with low levels of CCL21 expression had a fundamentally cytotoxic T-cell response, with greater production of interferon-γ and interleukins 2 and 4.45
Survival of Tumor CellsCXCL12 promotes survival of tumor cells expressing the receptor CXCR4 when grown under suboptimal conditions such as in the hypoxic environment commonly found in tumors.18 CXCL12 can also stimulate the production of metalloproteinases, which facilitate tissue invasion by the tumor cells.11 The interaction between CXCL9 and CXCR3 that occurs in tumor cells also potentiates their survival46 and the transendothelial migration of melanoma cells.47 In fact, high expression of both CXCR4 and CXCR3 by melanoma cells is a sign of poor prognosis in affected patients,48–50 although high-level expression of CXCR3 in circulating lymphocytes in patients with stage 3 melanoma is an indicator of a good immune response against the tumor.51 High levels of CXCL10 expression also reduce the proliferative and invasive capacity of melanomas.35 The role of the more recently described receptor CXCR7 remains to be fully determined. It is known to bind both CXCL12 and CXCL11 and appears to eliminate them, but its effects in melanomas are unclear.3
The effect of ligands for CXCR2 during tumor progression is complex. An intriguing observation is that chemokine signaling through CXCR2 induces senescence in the initial phases of tumor development but not in later phases, when it also has tumorigenic effects, perhaps due to the accumulation of genetic abnormalities in the tumor cells.52 Thus, high expression of CXCR1 and CXCR2 in melanoma cells confers an aggressive phenotype as a result of a greater proliferative, migratory, and growth capacity of the tumor.53,54 The role of CCL2, although not fully elucidated, also appears to be biphasic.55
Interestingly, when cells become isolated during the process of migration and dissemination, they become susceptible to a type of apoptosis known as anoikis, but expression of CXCR4 and CCR7 allows them to escape this process by reducing expression of the modifying factor bcl-2.56
Therapeutic TargetsThe confirmation that expression of certain chemokine receptors and their corresponding ligands influences the development of metastasis has led to the proposal of using antagonists, inhibitors, or inactivators of those receptors and ligands to prevent metastatic spread of melanoma. Thus, neutralization of the interaction of some chemokines with their receptors—such as CXCL12 with CXCR4 or CCL19 and CCL21 with CCR7—could be predicted to prevent the development of metastasis. Preclinical studies in melanoma and other cancers appear to confirm this hypothesis.4,19,57,58 The interaction between CXCL12 and CXCR4, however, has a wide range of important physiological roles, and as a result, may not represent a viable therapeutic target in patients with melanoma. Nevertheless, CXCR4 is the therapeutic target against which most treatments are directed in multiple myeloma and leukemia.3
There is evidence that expression of CXCL8 in melanoma functions as an autocrine or paracrine growth factor and promotes angiogenesis and tumor invasion through its interaction with the receptors CXCR1 and CXCR2.31 As a consequence, these chemokine receptors are potential therapeutic targets. Indeed, inhibitors of these receptors (SCH-479833 and SCH-527123) and also humanized neutralizing antibodies against them inhibit the proliferation, invasion, and metastatic growth of melanomas in immunodeficient mice.59 Other molecules that function as antagonists of these 2 receptors (repertaxin for CXCR1 and SB-225002 and SB-332235 for CXCR2) have been used in inflammatory conditions.31
Somewhat paradoxically, high endogenous production of CCL21 by melanoma cells causes immunosuppression, whereas exogenous provision of this chemokine has an effective antitumor effect.60 Care should therefore be taken when considering the use of CCL21 antagonists such as the recently proposed Chemotrap-1, which blocks in vitro and in vivo migration of melanoma cells towards the lymph vessels and inhibits in-transit metastasis.61
ConclusionsThe participation of chemokines and their receptors in the progression of melanoma is important, complex, and dynamic, and is dependent on both the characteristics of the tumor cells and on those of other cells present in the host microenvironment of the tumor. Cellular and molecular interactions determine whether a potentiating effect that favors tumor progression or an antitumor effect that favors regression or at least stabilization of the melanoma predominates. Given the essential role of interactions such as CXCR4-CXCL12 in the physiology of different organs and systems, care should be taken in any attempts to block their function until more information is available on the potential effects. Drugs targeting the chemokines and their receptors have begun to be used in some cancers and inflammatory conditions, and this will provide important information regarding the advantages and drawbacks of exploiting these interactions as therapeutic targets before they are used in patients with melanoma.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Monteagudo C, et al. Papel de las quimiocinas en la progresión del melanoma. Actas Dermosifiliogr.2011;102:498-504.