Secukinumab (SEC) and ustekinumab (UST) are monoclonal antibodies that have both been shown to be highly effective in the treatment of psoriasis,1 although in pivotal studies SEC achieved higher rates of efficacy.2 Their mechanisms of action differ in the target each one blocks in the inflammatory cascade: SEC is an interleukin (IL)-17 inhibitor, while UST blocks IL-12 and IL-23. Clinical trials have demonstrated the efficacy of SEC in patients previously treated with other systemic drugs, including monoclonal antibodies that inhibit tumor necrosis factor (TNF).3 However, to date there have been no publications about its effectiveness following treatment failure with UST.
Our aim in this study was to assess the efficacy of SEC in patients who had experienced primary or secondary treatment failure with UST, defined respectively as a failure to achieve a 75% reduction in baseline Psoriasis Area and Severity Index (PASI 75) at week 12 or the subsequent loss of such a response. The primary objective was to assess whether patients on SEC had achieved, by week 12, a 90% reduction in baseline PASI (a PASI 90 response), a Physician Global Assessment (PGA) of 1 or less (clear or almost clear), and a Dermatology Life Quality Index (DLQI) of 5 or less. The secondary objective was to assess whether the response was maintained at week 24.
We retrospectively evaluated 6 cases of patients with plaque psoriasis and an inadequate response to prior treatment with UST who were treated with the SEC regimen specified in the Summary of Product Characteristics (complete induction and maintenance with 300mg every 4 weeks). PASI, PGA, and DLQI were measured at baseline and at weeks 12 and 24.
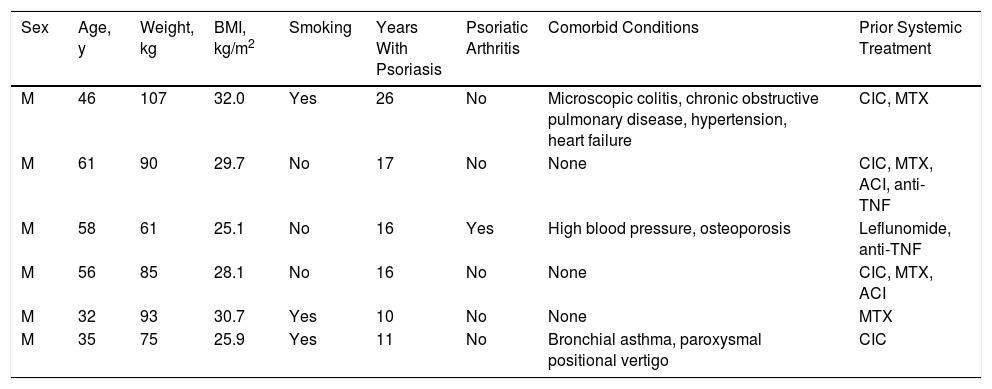
The clinical and epidemiological characteristics of the patients are shown in Table 1. We studied 6 men aged between 32 and 61 years (median, 51 years) with a history of psoriasis of more than 10 years duration (median, 16 years).
Clinical and Epidemiological Characteristics of the Patients.
| Sex | Age, y | Weight, kg | BMI, kg/m2 | Smoking | Years With Psoriasis | Psoriatic Arthritis | Comorbid Conditions | Prior Systemic Treatment |
|---|---|---|---|---|---|---|---|---|
| M | 46 | 107 | 32.0 | Yes | 26 | No | Microscopic colitis, chronic obstructive pulmonary disease, hypertension, heart failure | CIC, MTX |
| M | 61 | 90 | 29.7 | No | 17 | No | None | CIC, MTX, ACI, anti-TNF |
| M | 58 | 61 | 25.1 | No | 16 | Yes | High blood pressure, osteoporosis | Leflunomide, anti-TNF |
| M | 56 | 85 | 28.1 | No | 16 | No | None | CIC, MTX, ACI |
| M | 32 | 93 | 30.7 | Yes | 10 | No | None | MTX |
| M | 35 | 75 | 25.9 | Yes | 11 | No | Bronchial asthma, paroxysmal positional vertigo | CIC |
Abbreviations: ACI, acitretin; anti-TNF: monoclonal antibody targeting tumor necrosis factor; CIC: ciclosporin; M: man; MTX: methotrexate.
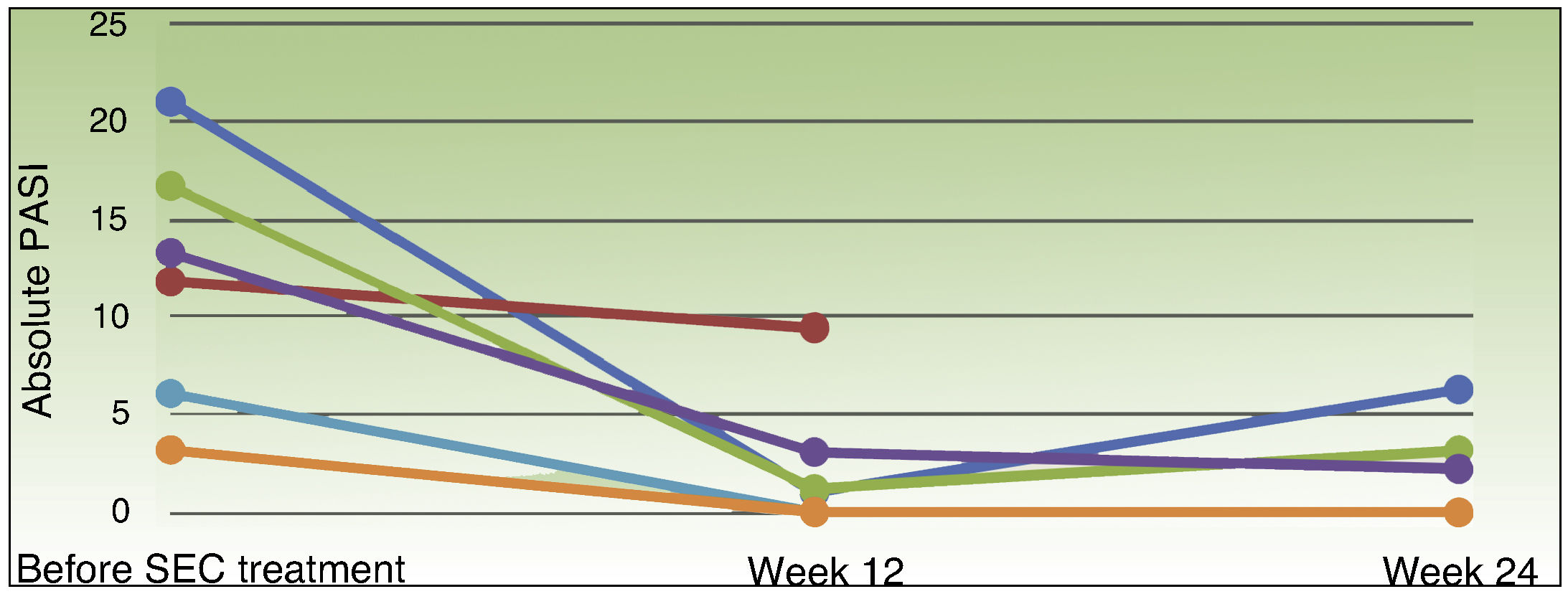
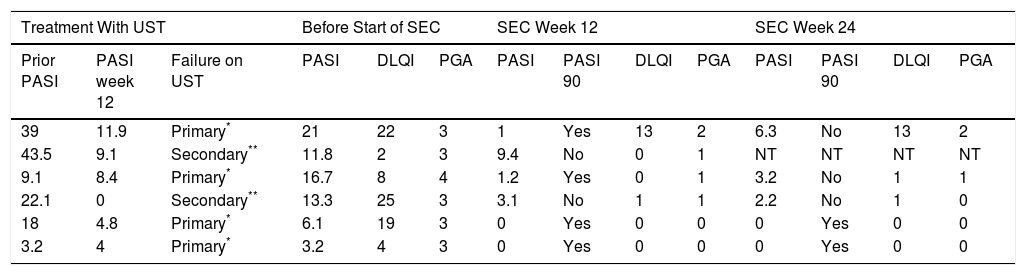
Table 2 shows the response to treatment with UST and SEC. Treatment failure with UST was primary in 4 patients and secondary in 2. The patients’ PASI scores before starting treatment with SEC ranged from 3.2 to 21 (median, 12.6), the PGA from 3 to 4 (median, 3) and the DLQI from 2 to 25 (median, 13.5). None of the patients received concomitant treatments in addition to SEC. All of the patients presented a reduction in disease activity at week 12 (Fig. 1): PASI 90 in 66.7% (4/6), and PGA and DLQI of 1 or less in 83.3% (5/6). SEC was withdrawn in 1 patient who did not achieve a PASI 50 response. At week 24, 33.3% (2/6) of the patients had a PASI 90 response, 66.6% (4/6) achieved PASI 75, 50% (3/6) had an absolute PASI of less than 3, and 66.6% (4/6) a PGA and DLQI of less than 1. No significant adverse events were observed during the 24 weeks of treatment with SEC.
Response to Secukinumab in Patients with Plaque Psoriasis Following Treatment Failure With Ustekinumab.
| Treatment With UST | Before Start of SEC | SEC Week 12 | SEC Week 24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior PASI | PASI week 12 | Failure on UST | PASI | DLQI | PGA | PASI | PASI 90 | DLQI | PGA | PASI | PASI 90 | DLQI | PGA |
| 39 | 11.9 | Primary* | 21 | 22 | 3 | 1 | Yes | 13 | 2 | 6.3 | No | 13 | 2 |
| 43.5 | 9.1 | Secondary** | 11.8 | 2 | 3 | 9.4 | No | 0 | 1 | NT | NT | NT | NT |
| 9.1 | 8.4 | Primary* | 16.7 | 8 | 4 | 1.2 | Yes | 0 | 1 | 3.2 | No | 1 | 1 |
| 22.1 | 0 | Secondary** | 13.3 | 25 | 3 | 3.1 | No | 1 | 1 | 2.2 | No | 1 | 0 |
| 18 | 4.8 | Primary* | 6.1 | 19 | 3 | 0 | Yes | 0 | 0 | 0 | Yes | 0 | 0 |
| 3.2 | 4 | Primary* | 3.2 | 4 | 3 | 0 | Yes | 0 | 0 | 0 | Yes | 0 | 0 |
Abbreviations: DLQI, Dermatology Life Quality Index; NT, treatment withdrawn due to lack of response. (failure to achieve PASI 50 by week 12); PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; SEC, secukinumab; UST, ustekinumab.
In this series of 6 patients in whom treatment with UST had not achieved an acceptable response, 4 patients achieved the primary treatment goal with SEC at week 12. Only 2 patients maintained a PASI 90 response at week 24 (the secondary objective). We used a PASI 90 response as an indicator in line with the current trend towards setting more demanding goals with new biologic therapies. However, most of the patients achieved a PASI 75 response and a PGA 1 or less at weeks 12 and 24, and reported significant improvement in quality of life, reflected by a reduction in DLQI in almost all cases.
The results obtained with SEC were not as good as those reported in the pivotal studies,2,3 a difference that may be due to the inclusion of patients who had previously failed to respond to several systemic therapies, an indication that their condition was difficult to manage. Another possible explanation might be found in the mechanism of action since by inhibiting IL-23, UST indirectly inhibits IL-17.4 Consequently, patients who had failed to respond to UST had already experienced some degree of blockade of the pro-inflammatory IL targeted by SEC without an acceptable clinical response. To further explore this hypothesis, it would be important to assess the response to SEC in patients who fail to respond to treatment with TNF antagonists, which do not inhibit IL-17. However, we found no publications relating to this clinical situation. In the pivotal trials of SEC, fewer than 10% of patients had previously failed to respond to anti-TNF therapy,2,3 and the data relating to this subgroup was unfortunately not analyzed.
Finally, we did not identify any predictors of response to SEC following treatment failure with UST.
The main strengths of this study were the use of multiple clinical response criteria (PASI, PGA and DLQI) before and during biologic therapy and follow-up of the response at 24 weeks. The main limitations were the retrospective nature of the study and the small number of patients included.
In conclusion, SEC can be an alternative treatment option in patients who have failed to respond to treatment with UST.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morgado-Carrasco D, Riera-Monroig J, Fustà-Novell X, Gibert MA. Respuesta a secukinumab tras fracaso terapéutico con ustekinumab en seis pacientes con psoriasis en placas. Actas Dermosifiliogr. 2018;109:565–567.