Recurrent aphthous stomatitis is a chronic inflammatory disease of the oral mucosa. It is characterized by painful mouth ulcers that cannot be explained by an underlying disease. Recurrent oral mucosal ulcers require a proper differential diagnosis to rule out other possible causes before recurrent aphthous stomatitis is diagnosed. The condition is common, with prevalence rates ranging from 5% to 60% in different series. Its pathogenesis is unknown, but multiple factors are considered to play a part. There are no standardized treatments for this condition and none of the treatments are curative. The goal of any treatment should be to alleviate pain, reduce the duration of ulcers, and prevent recurrence.
La aftosis oral recidivante es una enfermedad inflamatoria crónica de la mucosa oral. Se caracteriza por presentar úlceras dolorosas en la cavidad oral sin que se encuentre una enfermedad subyacente que lo justifique. Ante la aparición de úlceras recidivantes en la mucosa oral habrá que realizar un correcto diagnóstico diferencial y descartar otras causas antes de llegar al diagnóstico de aftosis oral recidivante. Se trata de una enfermedad frecuente, según la población estudiada se han documentado prevalencias entre el 5 hasta el 60%. Su patogenia es desconocida pero se considera multifactorial. El tratamiento no está estandarizado, y no hay un tratamiento curativo, se pretende disminuir el dolor durante el brote, acortar la duración del mismo y evitar la aparición de nuevas lesiones.
Recurrent aphthous stomatitis (RAS) is characterized by the appearance of painful, round, well-defined ulcers with erythematous borders and a grayish-yellow pseudomembranous base in the oral cavity of otherwise healthy patients. A burning sensation may precede the appearance of the ulcers by 2 to 48 hours.1
EpidemiologyRAS is the most frequent cause of ulcers on the oral mucosa. It affects 5% to 25% of the general population,2 although prevalence may vary from 5% to 60%3 depending on the study and on the population assessed, the diagnostic criteria applied, and environmental factors.
The peak age for onset of RAS is between 10 and 19 years, and although the condition is less frequent in adults, it may persist throughout a person’s life. No sex differences in prevalence have been recorded.4
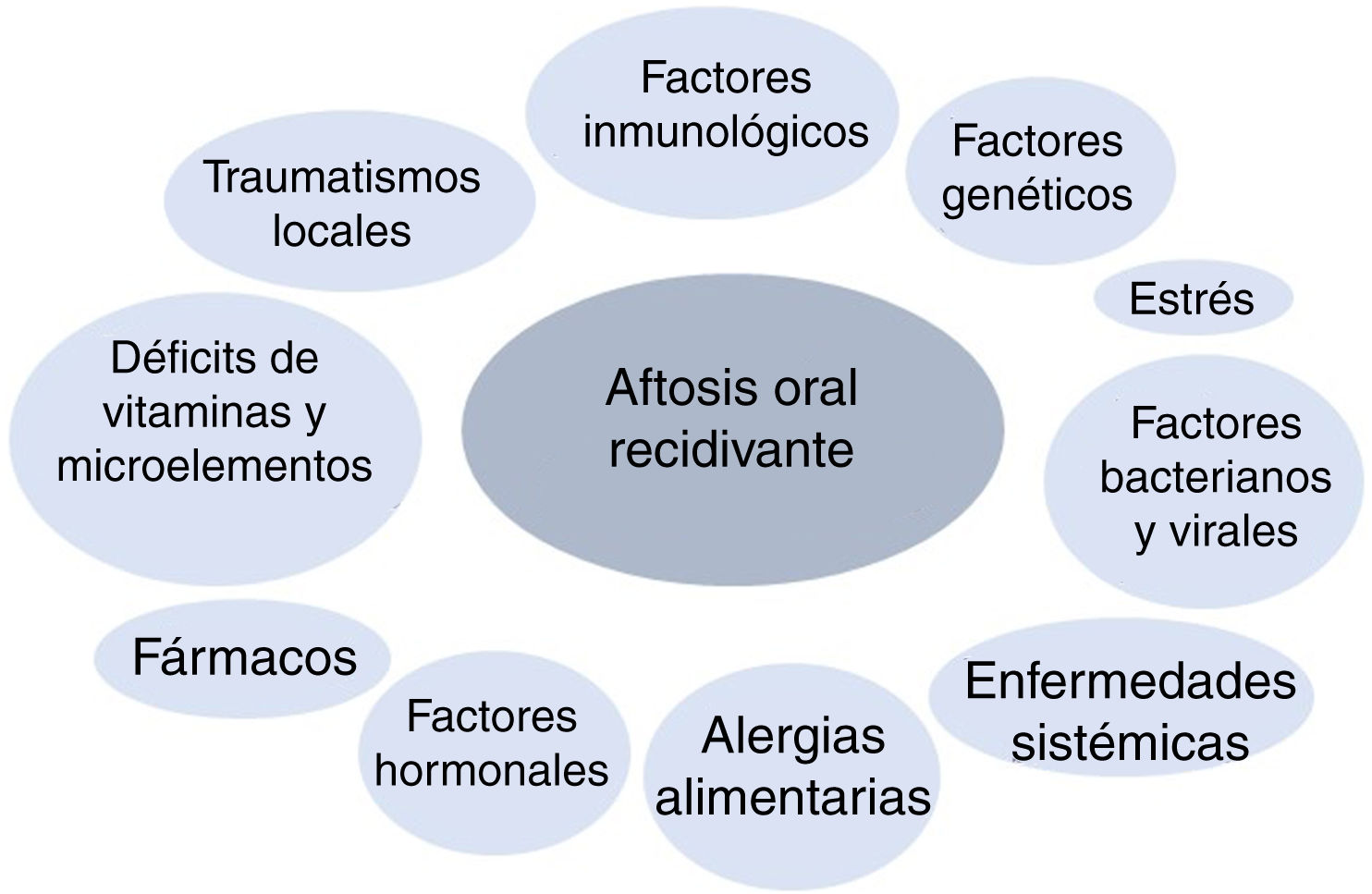
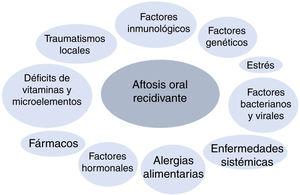
PathogenesisRAS belongs to the family of chronic inflammatory diseases of the oral mucosa. Its etiology and pathogenesis are unknown, although it is considered a multifactorial disease, and various triggers have been reported (Fig. 1). In genetically predisposed patients, the effect of specific factors is thought to initiate a proinflammatory cytokine cascade targeting specific areas of the oral mucosa.5
- •
Genetic factors: Heredity plays a key role in the development of RAS. The probability of having RAS increases if one or both parents have had the disease; in 24%-46% of cases, patients have a family history.6,7 Moreover, patients with a family history experience recurrences much more often and have a more severe clinical picture.8–10
The incidence of human leukocyte antigen (HLA) A33, HLA-B35, HLA-B81, HLA-B12, HLA-B51, HLA-DR7, and HLA-DR5 is greater in patients with RAS than in healthy controls.5
Genetic risk factors also modify a person’s susceptibility to RAS. These include various DNA polymorphisms throughout the human genome, especially those associated with the metabolism of interleukins (IL) (e.g., IL-ß, IL-2, IL-4, IL-5, IL-6, IL-10, and IL-12), interferon γ, and tumor necrosis factor (TNF) α.11–20
- •
Local injury: Local injury is considered a causal agent in genetically predisposed individuals21,22 and predisposes to RAS, with early cellular inflammation and edema, as well as increased viscosity of the extracellular matrix of the oral submucosa.23 Not all local injuries lead to RAS, since persons who wear dentures are not at an increased risk.24 Smoking has been reported to act as a protective factor with respect to RAS.25,26
- •
Bacterial and viral factors: Various reports have attempted to establish an association between RAS and different microorganisms, including bacteria of the genus Streptococcus, especially Streptococcus sanguinis 2A,27Helicobacter pylori,28Lactobacillus,29 and Epstein-Barr virus.30 However, the results to date have not shown a clear causal relationship.
- •
Stress: Stressful life events may trigger new lesions in predisposed patients. One study concluded that mental stressors were more associated with RAS than physical stressors and that stressful life events were more associated with the onset of episodes than with the duration of the episodes.21 Similarly, there have been cases of diseases, such as Behçet disease, that progress with aphthous ulcers and that worsen after considerable emotional stress.31
- •
Food allergy: Allergy is thought to be a cause of RAS. Hypersensitivity to specific substances, oral microorganisms such as S sanguinis, and heat shock proteins have been proposed as causal factors, although there is no evidence to date that these are a key cause of the disease.32,33
- •
Vitamin and micronutrient deficiencies: Low levels of iron, folic acid, zinc, and vitamins B1, B2, B6, and B12 have been reported.34 Sometimes, these deficiencies are associated with underlying diseases, such as malabsorption and gluten enteropathy.
- •
Immunologic factors: In patients with RAS, the functioning of the immune system is modified in response to an as yet unknown trigger (e.g., bacterial/viral antigens and stress). Both the innate and acquired immune responses (humoral and cellular) are altered in patients with RAS. Many authors believe that the type 1 helper T (TH1) response plays the most important role in the development of the disease.15,35
- •
Underlying systemic diseases: RAS appears more frequently in patients with inflammatory bowel disease (Crohn disease and ulcerative colitis) and in celiac disease.6,36,37 This association could result from nutritional deficiency, which is a frequent complication of these diseases. RAS is also more frequent in patients infected with the human immunodeficiency virus, probably in association with an abnormal CD4+/CD8+ ratio and a reduced neutrophil count.38,39
- •
Hormonal factors: An association has been reported between the appearance of aphthous ulcers and the menstrual cycle. Ulcers are more frequent during the luteal phase or in the menopause and less frequent during pregnancy and in women taking hormonal contraceptives.40
- •
Drugs: There are reports of oral aphthous ulcers triggered by drugs. One case-control study associated an increased risk of RAS with medication, especially nonsteroidal antiinflammatory drugs and ß-blockers.42 Nicorandil, calcineurin, and mTOR inhibitors have also been associated with severe oral ulcers.43–45
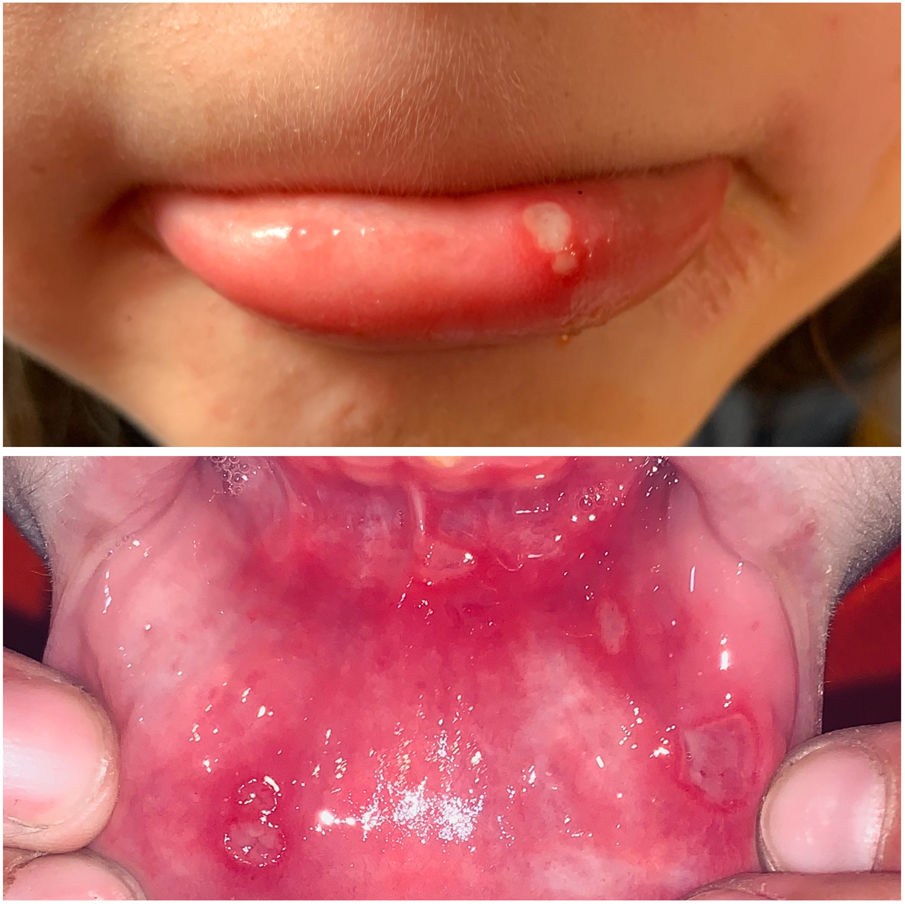
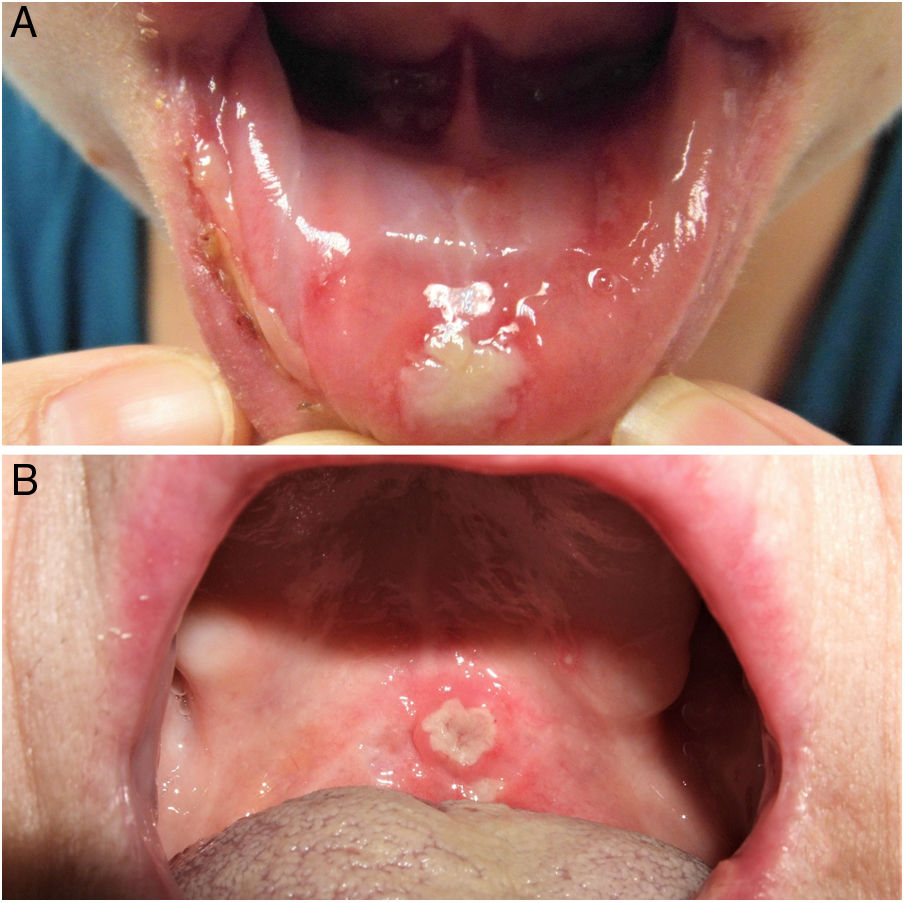
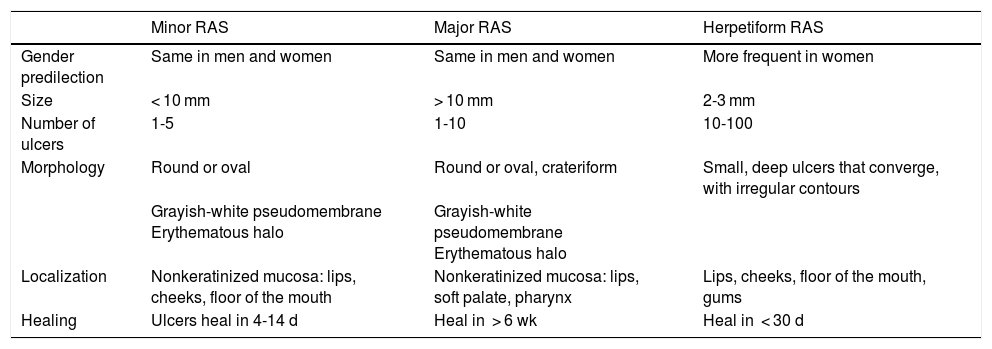
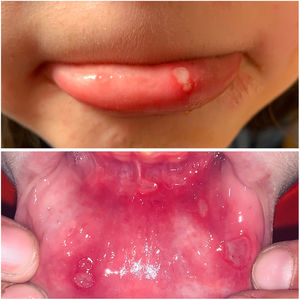
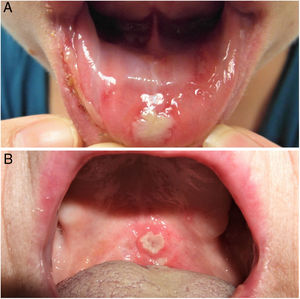
The 3 clinical forms of RAS—major, minor, and herpetiform—differ in their morphology, distribution, severity, and prognosis. Table 1 summarizes the main differences. Despite these differences, all 3 types of RAS have a significant impact on patients’ quality of life and interfere with activities of daily living.46 Minor RAS (Fig. 2) is the most common presentation, affecting 80% of patients. It progresses without scarring, unlike major RAS (Fig. 3), in which ulcers take longer to heal and which progresses with scarring and even residual synechiae. Herpetiform RAS is the least common type. In this case, there could be up to 100 ulcers that can coalesce, leading to larger ulcers with irregular borders.
Clinical Classification.
| Minor RAS | Major RAS | Herpetiform RAS | |
|---|---|---|---|
| Gender predilection | Same in men and women | Same in men and women | More frequent in women |
| Size | < 10 mm | > 10 mm | 2-3 mm |
| Number of ulcers | 1-5 | 1-10 | 10-100 |
| Morphology | Round or oval | Round or oval, crateriform | Small, deep ulcers that converge, with irregular contours |
| Grayish-white pseudomembrane Erythematous halo | Grayish-white pseudomembrane Erythematous halo | ||
| Localization | Nonkeratinized mucosa: lips, cheeks, floor of the mouth | Nonkeratinized mucosa: lips, soft palate, pharynx | Lips, cheeks, floor of the mouth, gums |
| Healing | Ulcers heal in 4-14 d | Heal in > 6 wk | Heal in < 30 d |
Abbreviation: RAS, recurrent aphthous stomatitis.
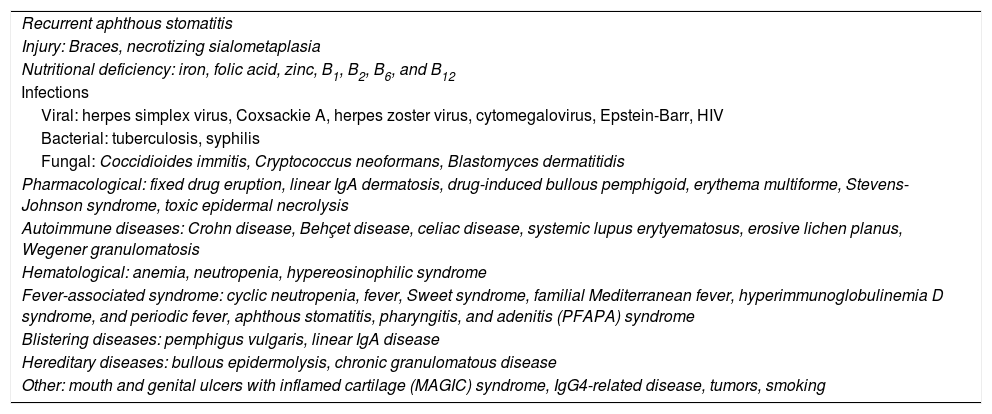
Table 2 shows the main causes of acute and chronic ulcers in the oral mucosa.
Differential diagnosis of acute and chronic oral ulcers.
| Recurrent aphthous stomatitis |
| Injury: Braces, necrotizing sialometaplasia |
| Nutritional deficiency: iron, folic acid, zinc, B1, B2, B6, and B12 |
| Infections |
| Viral: herpes simplex virus, Coxsackie A, herpes zoster virus, cytomegalovirus, Epstein-Barr, HIV |
| Bacterial: tuberculosis, syphilis |
| Fungal: Coccidioides immitis, Cryptococcus neoformans, Blastomyces dermatitidis |
| Pharmacological: fixed drug eruption, linear IgA dermatosis, drug-induced bullous pemphigoid, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis |
| Autoimmune diseases: Crohn disease, Behçet disease, celiac disease, systemic lupus erytyematosus, erosive lichen planus, Wegener granulomatosis |
| Hematological: anemia, neutropenia, hypereosinophilic syndrome |
| Fever-associated syndrome: cyclic neutropenia, fever, Sweet syndrome, familial Mediterranean fever, hyperimmunoglobulinemia D syndrome, and periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome |
| Blistering diseases: pemphigus vulgaris, linear IgA disease |
| Hereditary diseases: bullous epidermolysis, chronic granulomatous disease |
| Other: mouth and genital ulcers with inflamed cartilage (MAGIC) syndrome, IgG4-related disease, tumors, smoking |
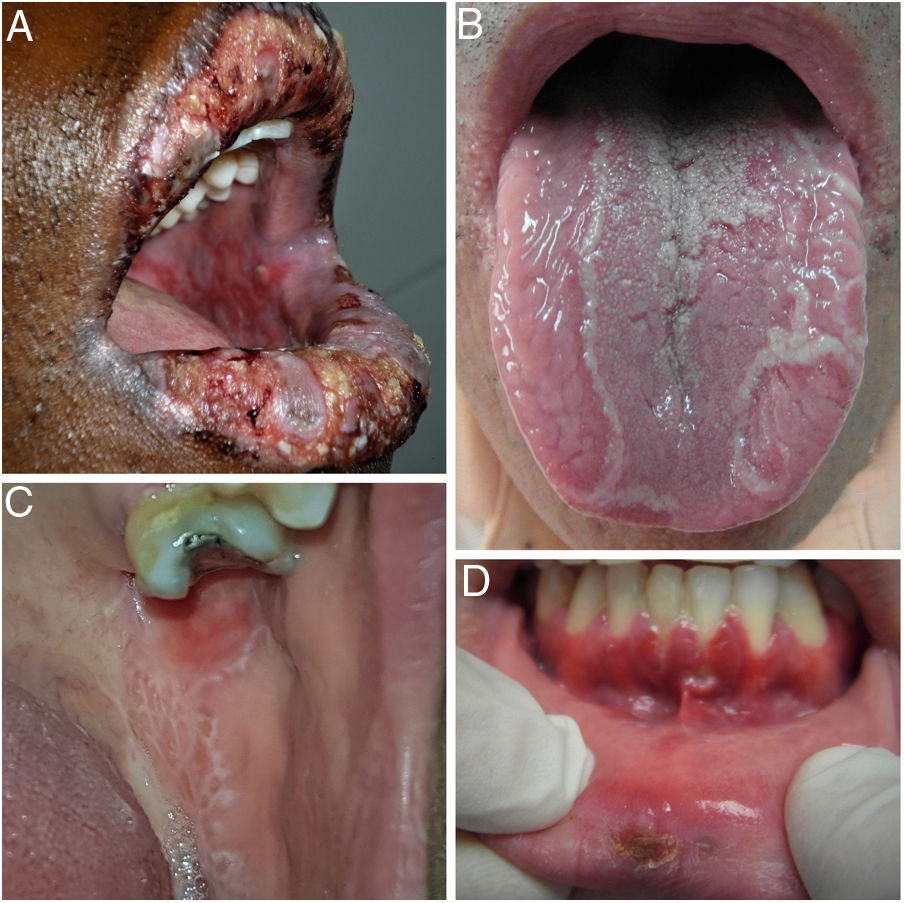
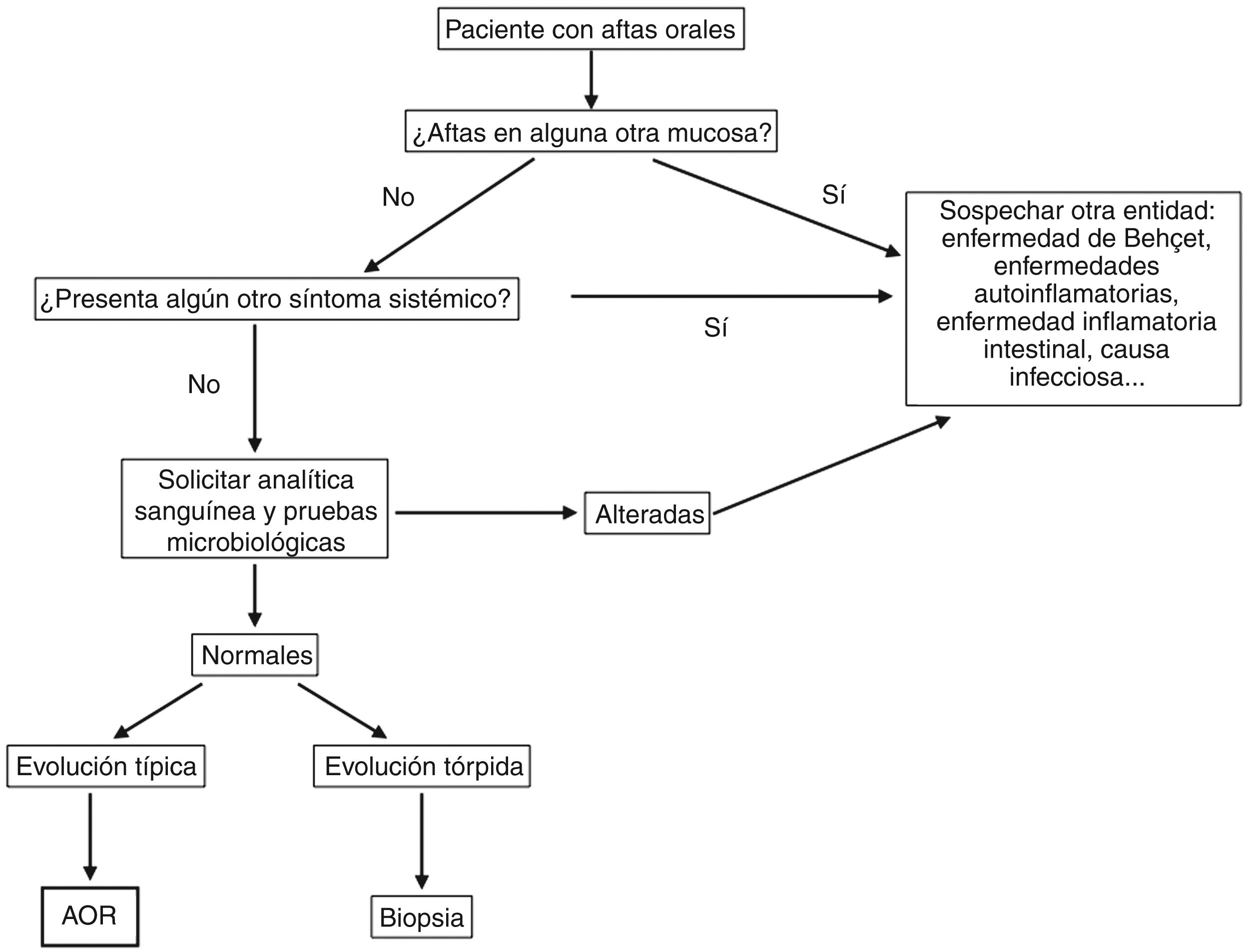
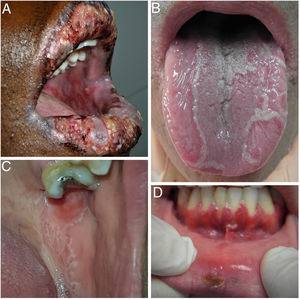
Given the complex differential diagnosis of RAS, a meticulous history must be taken to correctly guide the diagnosis. Before confirming a diagnosis, we must rule out other clinical pictures in which ulcers are one of the most frequent signs (Fig. 4).
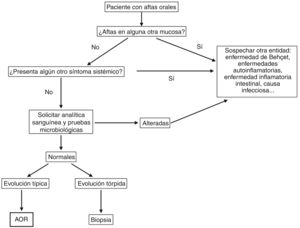
DiagnosisDiagnosis of RAS is based on the clinical history and a physical examination. However, we must always rule out an underlying systemic cause when ulcers appear for the first time, especially in adults.47,48Fig. 5 shows a diagnostic algorithm for patients with mouth ulcers.
The recommended additional tests include the following:
- •
Blood tests: complete blood count, iron, ferritin, folic acid, zinc, magnesium, and vitamins (B1, B2, B6 y B12). Testing should also include transglutaminase and endomysial antibodies to rule out celiac disease, as well as antinuclear antibodies. Furthermore, some studies have shown an association between RAS and positive titers for antigastric parietal cell antibody, and antithyroglobulin antibody, and antithyroid microsomal antibody.49
- •
Microbiological tests: Tzanck smear test or polymerase chain reaction assay for herpes virus and culture of fungi and bacteria.
- •
Skin biopsy: The 3 indications for a skin biopsy are as follows50:
- a
Ulcer of unknown origin that persists for more than 2 weeks with no signs of healing.
- b
Ulcer of probable origin (after the corresponding diagnostic tests) that does not respond after 2 weeks of appropriate treatment.
- c
Ulcer brought about by a trigger(s) that does not heal within 2 weeks of these factors being ruled out.
Punch or incisional biopsy: The specimen must be taken from the border of the lesion, including the area of the ulcer and the perilesional mucosa.
Histopathology study of the ulcers: The histopathology image reveals a leukocytic infiltration, which may vary depending on the duration and severity of the disease. In the initial phases, which precede formation of the ulcers, we mainly see an inflammatory infiltrate comprising T lymphocytes and monocytes. We can also see isolated mast cells and plasma cells, which accumulate below the basal layer. More advanced stages are characterized by a predominance of polymorphonuclear leukocytes in the center of the ulcer and mononuclear cells on the periphery.5 Consistent with Poulter and Lehner,51 this type of inflammation is not specific of RAS and can be seen in other ulcers such as erythema multiforme, Behçet disease, lupus erythematosus, and traumatic ulcers.
TreatmentNo definitive curative treatment for RAS has been established to date. Therefore, the primary objectives of treatment are to relieve pain, accelerate healing, and reduce the frequency and severity of episodes of RAS.52
As mentioned above, recurrent ulcers on the oral mucosa require a correct differential diagnosis. We must also rule out associated systemic diseases and other treatable causes before making a diagnosis of RAS and considering treatment.
The approach to therapy should be based on the severity of symptoms, the frequency and duration of the outbreaks, the clinical history, and the patient’s ability to tolerate medication. Patients with isolated episodes of simple RAS that last only a few days require no more than topical treatments for relief of pain and a series of general measures, mainly good oral hygiene. Systemic therapy is indicated in patients who experience multiple episodes of RAS and/or severe cases that involve intense pain and difficulty eating and do not respond to topical medication.52,53
Despite the frequency of this condition, few high-quality studies have appropriately evaluated treatment of RAS. Therefore, there is no standard therapy.52 The multiple topical and systemic treatments used have had varying degrees of success.
General MeasuresOral hygiene: It is important to ensure appropriate oral hygiene and to avoid injury, since this leads to mouth ulcers.31,54 We recommend using a soft toothbrush, toothpaste that does not contain sodium lauryl sulfate (grade of recommendation [GR], A; level of evidence [LE], 1B),55,56 and an alcohol-free mouthwash.31,55,57
Eating: No relevant studies have appropriately addressed the role of diet in the management of RAS. In general, we should try to avoid products that are frequently associated with triggering flare-ups, especially if the patient reports an association with the products.54,57
Supplements: It is necessary to rule out nutritional deficiencies (e.g., vitamin B12, folic acid, iron, zinc) in patients with RAS, since in these cases, patients improve when they receive appropriate treatment (GR, C; LE, 4). Furthermore, one study showed that sublingual vitamin B12 at 1000 μg/d for 6 months could reduce the number of flare-ups and relieve pain in all patients with RAS independently of previous levels (GR, B; LE, 2B).56–58 Other studies have shown an improvement with ω-3 at 1000 mg/d for 6 months (GR, B; LE, 2B).59 In contrast, supplements with other vitamin complexes in patients without nutritional deficiency have not led to an improvement in symptoms or a reduction in the number of lesions (GR, B; LE, 2B).52,57,59,60
Topical TreatmentsTopical anesthetics and barrier agents: These agents provide pain relief. They should be applied several times per day, preferably half an hour before meals and before teeth cleaning in order to facilitate their action and at bedtime. They can be used in combination with other treatments such as topical corticosteroids or amlexanox. The main protective and anesthetic agents include the following:
- •
Lidocaine cream 1%, gel 2%, and spray: these are applied directly on the surface of the ulcers or in the form of mouthwashes (GR, B; LE, 2A).60
- •
Benzocaine gel 20%: local anesthetic that relieves pain and reduces inflammation (GR, B; LE, 2A).61
- •
Sucralfate suspension: a complex of aluminum hydroxide and sucrose sulfate complex that forms a protective barrier against ulcers. Several studies recommend mouth washing with 5 mL for 1-2 minutes 4 times daily (after teeth cleaning and at bedtime). This substance relieves pain, speeds up healing, and lengthens the interval between flare-ups (GR, B; LE, 2A).62,63
Topical antiinflammatory and antiseptic agents: These help to prevent superinfection by bacteria and fungi and improve oral hygiene.
- •
Triclosan 0.15% in ethanol and zinc sulfate: Administered as 3 mouthwashes per day, this agent reduces the number of ulcers and the intensity of pain and increases the ulcer-free interval (GR, A; LE, 1B).63
- •
Chlorhexidine 0.12%-0.2% in oral solution: 5 mL in rinses for 1-2 minutes 4 times daily (after teeth cleaning and at bedtime).60 Some studies report it to be less efficacious than sucralfate suspension (GR, B; LE, 2B).62
- •
Diclofenac 3% in hyaluronic acid gel 2.5%: This agent proved superior to lidocaine in gel for reducing pain after 2-6 hours (GR, B; LE, 2A).60,63
Topical corticosteroids: These are the first-line agents for RAS60 and can be combined with topical anesthetics, antiseptics, and barrier agents. They provide pain relief and reduce the duration and frequency of flare-ups, although they take several days to exert an effect. They are more effective if used from the onset of the episode and are applied several times per day, preferably after teeth cleaning and at bedtime. The patient should be advised not to eat at least during the following half hour.62,64 The available options are as follows:
- •
Triamcinolone acetonide 0.1% in Orabase: This is applied on the lesions 3-4 times per day. It has proven to be a safe and effective treatment (GR, B; LE, 2B).60
- •
Dexamethasone solution (0.5 mg/5 cc) or ointment: Rinsing every 5 minutes, 3-4 times per day or applying the ointment on the ulcers 3 times per day has proven to be effective and safe (GR, A; LE, 1B).59,64
- •
Clobetasol 0.05% in gel, ointment, or Orabase: The agent is applied to the lesions 2-3 times per day. As this is the most potent corticosteroid, it is reserved for the most severe cases.60
Amlexanox 5%: Topical inflammatory agent that, when applied on the lesions in the form of a 5% paste 4 times per day (after meals and at bedtime) has proven to be effective for the management of pain and for speeding up healing in several studies (GR, B; LE, 2B).52,56,61,65 Amlexanox 5% is one of the most cost-effective topical treatments, together with triamcinolone acetonide.61
Cauterization: Applied with hydrogen peroxide 0.5% solution or silver nitrate 1%-2%, this approach reduces pain and speeds up healing (GR, B; LE, 2B).56,57
Other topical treatments: Many other topical treatments have been used, including tetracyclines in mouthwash, doxycycline in denture adhesive, nicotine gum, liquid diphenhydramine mouthwash, or camel thorn distillate, most of which have been reported in poor-quality studies, with disparate results and no evidence to recommend their use.52,57,66,67
Systemic TreatmentsPatients who experience severe and/or frequent episodes of RAS that are refractory to general care and to topical treatments (see above) should consider adding a systemic treatment. This is selected based on the severity of symptoms, comorbid conditions, and the patient’s tolerance and preferences.
First-LineOral corticosteroids: Oral corticosteroids have been used effectively in long regimens at lower doses, for example, oral prednisone at 5 mg/d for 3 months (GR, B; LE, 2A),67 and in shorter regimens, with doses of 20 to 40 mg/d for 4-7 days, with subsequent gradual reduction of the dose. This leads to relief of pain, faster healing, and a reduction in the number of episodes (GR, B; LE, 2A).52,54,56,57
Second-LineAlternative systemic agents should be considered in patients who do not respond to intermittent therapy with systemic corticosteroids, patients who require frequent or longer courses of corticosteroids, and patients who cannot undergo treatment with corticosteroids for other reasons.
Colchicine: Colchicine is an antimitotic drug with specific immunomodulatory and antifibrotic effects. It has been used at 0.5 to 2 mg/d, with various results.52,56,57 In some publications, it is considered a systemic drug of choice at doses of 1-2 mg/d over long periods, depending on the severity of symptoms and tolerance.56,57,68 In contrast, after an analysis of published studies, the 2012 Cochrane review52 concluded that this drug was not useful in comparison with oral corticosteroids for the treatment of RAS, since its efficacy rates are equal to or lower than those of corticosteroids, although it has a greater rate of adverse effects (mainly affecting the gastrointestinal tract) (GR, B; LE, 2A).52,69,70
Thalidomide: Thalidomide has been used at 50 to 100 mg/d for several months with good results.56,57,70 A study performed in Brazil showed it to be more effective and better tolerated than dapsone, colchicine, and pentoxyphillin.71 We must remember that this drug is teratogenic and can cause sleepiness, paresthesia, and irreversible peripheral neuropathy. Therefore, patients should be carefully selected and informed. It is also advisable to take a history and perform an examination at all follow-up visits to rule out signs of peripheral neuropathy. If this condition is suspected, treatment should be discontinued and an electromyogram ordered (GR, B; LE, 2B).56,57,70
Dapsone: Dapsone reduces the number and size of the ulcers.63 It is usually started at 25-50 mg/d, increasing to a maximum of 150 mg/d depending on the response and on tolerance. Glucose-6-phosphate dehydrogenase should be determined before starting therapy with dapsone (GR, B; LE, 2A).60,67
Montelukast: Montelukast is a leukotriene inhibitor that, in some trials, has been shown to improve pain and accelerate healing of mouth ulcers, as well as reduce the appearance of new lesions with a dose of 10 mg/d for 1 month, followed by 10 mg every 2 days for a further month.72 Montelukast is less efficacious than prednisone, although it has fewer adverse effects and is very well tolerated. Therefore, it could prove to be a good option for long-term treatment (GR, B; LE, 2B).52,56,72
Clofazimine: Clofazimine is an antimicrobial agent that has been used at 100 mg/d for 30 days followed by 100 mg every other day for 6 months. In a study with a high risk of bias, it improved symptoms and reduced the number of flare-ups compared with colchicine (GR, B; LE, 2B).52,69
ß-Glucans: ß-Glucans comprise a group of polysaccharides found in some bacteria, plants, and fungi. They have been used at doses of 10 mg of 1,3-1,6 ß-glucan twice daily, with an improvement in the severity of the ulcer compared with placebo.52,73 Evidence in favor of or against this approach in RAS is insufficient (GR, B; LE, 2B).52
Pentoxifylline: This nonselective phosphodiesterase inhibitor with hemorheological properties specifically inhibits production of TNF-alfa and possibly production of some other type 1 helper T cells and proinflammatory cytokines such as IL-1ß, which are thought to play an important role in the development of RAS. Pentoxifylline has been used at 400 mg/8 h, with improvement of the lesions, although these recur when the drug is discontinued. Evidence in favor or against its use is insufficient (GR, B; LE, 2A).52,63
Levamisole: Levamisole is an anthelmintic and immunomodulatory agent.60 Results from trials with doses of 50 mg/8 h for 3 to 11 days per flare-up for at least 6 months show disparate efficacy outcomes, with insufficient evidence in favor of or against its use (GR, B; LE, 2A).52,62
Doxycycline: Doxycycline at 20 mg/12 h revealed no differences with respect to placebo in a study with a high risk of bias (GR, B; LE, 2A).52
Biological TreatmentsAnti-TNF-alfa: Data from case series show that anti-TNF alfa agents (etanercept, adalimumab, infliximab, and golimumab) have been used successfully for treatment of severe and recalcitrant RAS.62,74 This seems to be a specific class effect, with no significant differences between the anti-TNF agents used. Furthermore, it seems that failure with any of these drugs does not imply the lack of a response to other anti-TNF agents (GR, C; LE, 4).62,74 These drugs should be chosen based on disease severity, efficacy, potential adverse effects, and costs.
Other TreatmentsCO2, Nd:YAG, and diode laser: Some studies showed that laser therapy had similar or superior efficacy to topical corticosteroids,67 with immediate relief of pain and accelerated healing of the ulcers (GR, B; LE, 2A).53,57,75
Apremilast: Apremilast is an oral phosphodiesterase-4 inhibitor. In one published case of major RAS that was refractory to multiple topical and systemic treatments, 6 weeks’ treatment with apremilast (loading dose of 10 mg/d increasing progressively to 30 mg/12 h) led to complete resolution of the lesions, with no recurrences after 1 year of treatment (GR, C; LE, 4).76
Bee propolis: This resinous material is produced by bees and is obtained from the buds of poplars and conifers. One study with a high risk of bias showed that a daily capsule of 500 mg taken over 6 months led to a reduction in the number of outbreaks. However, evidence was insufficient to recommend or not recommend its use (GR, B; LE, 2A).52,77
Homeopathy: One study with a high risk of bias concluded that homeopathy could improve pain and accelerate the cure of ulcers, without there being sufficient evidence for recommending or not recommending its use (GR, B; LE, 2A).52,78
Traditional Chinese medicine: Some traditional therapies have been used for hundreds of years. A recent review tried to evaluate the scientific aspects of this approach by evaluating several of the treatments used, such as Liuwei Dihuang pills (composed of Cornus officinalis, Rehmannia glutinosa, Rhizoma dioscoreae, Cortex moutan radicis, Poria cocos, and Alisma plantago-aquatica), bergamot, Qing Wei powder, and Yiqing capsules. The authors concluded that some treatments in traditional Chinese medicine may be effective and safe for the treatment of RAS, although high-quality studies would be necessary to confirm these findings. Evidence to recommend their use is insufficient (GR, B; LE, 2B).79
Several publications address the drugs used in the treatment of oral aphthous ulcers that manifest in systemic diseases, such as Behçet disease, but not in RAS per se. These include azathioprine, methotrexate, ciclosporin, and interferon-alfa. Given that neither their efficacy with respect to the etiology and pathogenesis of aphthous ulcers nor their role in RAS has been studied, we decided not to include these drugs in order to avoid confusion.80–83
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez J, Conejero C, Conejero R. Aftosis oral recidivante. Actas Dermosifiliogr. 2020;111:471–480.