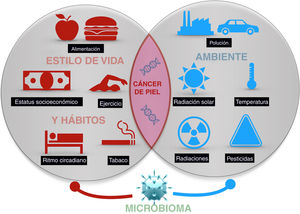
Skin cancer is the most frequent type of cancer in humans. While exposure to solar radiation is the most widely known and relevant causal factor, the different degrees of individual risk have not been fully elucidated. Epidemiological studies show how the risk of skin cancer is affected by other types of radiation, (eg, ionizing radiation), pesticides, particulate matter in air pollution, toxins (eg, arsenic) in water and some foods. Some living entities, such as polyomavirus and human papillomavirus, can also cause specific types of cancer. Lastly, lifestyle factors such as stress, sleep, and exercise may play a role, although only a few studies shed light on these factors. The abovementioned factors make up the exposome of skin cancer, that is, the set of environmental exposures that, together with the genome and microbiome, determine the onset of disease.
El cáncer de piel es el más frecuente del ser humano. Aunque la exposición a la radiación solar constituye el factor causal más conocido y relevante, existe una variación en el riesgo individual no explicada completamente. Diferentes estudios epidemiológicos muestran la influencia de otras radiaciones, como las ionizantes, los pesticidas, las partículas de la polución, o los tóxicos contenidos en el agua o algunos alimentos como el arsénico, en el riesgo del cáncer de piel. Además, algunos agentes vivos como los poliomavirus o el VPH son agentes etiológicos de algunos tipos concretos de cáncer cutáneo. Por último, algunos factores asociados al estilo de vida, como el estrés, el sueño, o el ejercicio podrían influir, aunque son muy escasos los estudios que aporten luz en estas áreas. Todo ello constituye el exposoma del cáncer cutáneo, el conjunto de exposiciones ambientales de un ser humano a lo largo de la vida que, combinados con el genoma y el microbioma, determinan la aparición del mismo.
The concept of exposome refers to the totality of environmental exposures that individuals experience during their lives and underlies a new approach to study of the role of the environment in human health.1 Linked to this concept, the EXPOsOMICs project has the aim of assessing environmental exposures, mainly environmental pollution and water contaminants, using omic technologies that can look for associations between exposure data and biochemical and molecular changes. The results will enhance our understanding of how contaminants influence the risk of developing both chronic diseases and different types of cancer.2
Skin cancer is the most frequent type of cancer in humans. As the skin is our most external organ and, therefore, the one in closest contact with the environment, it is doubtlessly the most exposed to the effects of what is going on in our surroundings. Of all possible types of exposure, ultraviolet (UV) radiation from sunlight has been recognized as the primary causal agent of skin cancer.3 However, in recent years, there is mounting evidence that air pollution, contaminants in water and food, and lifestyle can also influence development of the cancer. In a holistic concept of health, and taking into account the interaction between the psyche and nervous, endocrine, and immune systems, it is increasingly necessary to consider the influence of stress or sleep on the onset of cancer.
The present article aims to review available evidence on those external factors related to the onset of skin cancer of any type (Fig. 1).
RadiationUV radiation, particularly in individuals with fair skin, is the main cause of skin cancer, particularly keratinocyte skin cancer and melanoma.
Both type B UV (UVB) and type B (UVA) radiation are considered as human carcinogens.3 It is known that UV radiation participates in the onset of cancer, as UVB radiation induces direct DNA damage that gives rise to the formation of thymine dimers; UVA also induces direct and indirect DNA damage through the production of reactive oxygen species (ROS) leading to simple or double-chain breaks and DNA-protein crosslinks, and through oxidation to form 8-oxoguanine.4 This DNA damage induces p53 activation that can activate cell repair through nucleotide excision or drive the cell to apoptosis.5 If the p53 gene undergoes a mutation due to UV radiation, this process does not occur giving rise to clonal keratinocyte and melanocyte expansion with damaged DNA and to skin carcinogenesis. Added to this, UV radiation induces Langerhans cell depletion in the epidermis with the corresponding decreased skin immune surveillance and production of inflammatory cytokines, a state that favors the development of skin cancer.6
Epidemiological studies have shown that living in geographic areas with high levels of UV radiation is associated with an increased risk of skin cancer7; squamous cell carcinoma (SCC) is more related to cumulative or occupational exposure whereas basal cell carcinoma (BCC) and melanoma appear more related to intense sporadic exposure and a history of sunburn.8
The use of sunlamps increases the risk of developing both BCC and SCC proportionally with the extent of exposure, when usage occurred during adolescence.9 The use of sunlamps has been associated with an increased risk of developing melanoma, depending on the number of sessions and use at a young age (< 35 years).10
Ionizing radiation has also been established as a cause of nonmelanoma skin cancer (NMSC). Thus, occupational exposure in radiology technicians to low to moderate doses of radiation can increase the risk of BCC, particularly in individuals with light-colored eyes and fair hair.11 An increased risk is also observed in those who undergo radiotherapy.12
Cosmic radiation has been proposed as an agent that may induce melanoma and BCC in pilots and cabin crew. This group has twice the incidence of melanoma compared with the general population and there is a relationship with the number of flight hours and therefore with cumulative radiation exposure.13 Nevertheless, there is some doubt as to whether the lifestyle of this group of professionals may also have an effect.14
Between 1950 and 2011, an increase of 13% was detected in erythematous radiation due to decreased ozone levels in 9 Spanish cities.15 The 1987 Montreal protocol banned the emission of chlorofluorocarbons leading to a decrease in the levels of these agents over the Antarctic, with an associated decrease of 20% in the resulting ozone depletion.16 This decrease without doubt can have repercussions for the amount of UVB radiation that we receive, decreasing exposure and resulting, perhaps in the future, in a decrease in the incidence of skin cancer.
TemperatureHeat can also be a risk factor for skin carcinogenesis. In both cell cultures and experimental animals, administration of repeat doses of UVB and heat (39 °C) reduces apoptosis, deactivating p53-mediated response to stress and inducing expression of sirtuin 1 (SIRT1). Therefore, both environmental factors act synergistically to allow the survival of cells with damaged DNA.17
However, this interaction is not clear cut. A recent study has shown that if nude mice are first exposed to heat and then to UVB radiation, tumor onset is delayed and, in addition, the tumors that developed were smaller than those in mice treated only with UVB or with UVB followed by heat.18
PollutionIt is estimated that air pollution is responsible for approximately 7 million deaths worldwide every year.19 Its association with lung cancer is clear; however, its association with other cancers, such as skin cancer, has not been established.
The main air pollutants are polycyclic aromatic hydrocarbons (PAHs) (including benzopyrenes), which are derived mainly from automobile combustion and combustion of organic materials, including cigarettes. PAHs are the causal agents of scrotum cancer.20 In terms of the skin, it has been shown that applying a mixture of PAHs chronically to the skin of nude mice induces papilloma and SCC formation,21 whereas 7,12-dimethylbenzanthracene is able to induce lymphomas in hamsters.22
Particulate material (PM) suspended in the atmosphere, formed of a complex mixture of solid particles and liquid drops, are of prime importance as harmful agents for the skin. These are able to penetrate the follicles or enter the skin via a transdermal route.23 PM stimulates the production of ROS, such as the superoxide anion or hydroxyl radical, and favors the secretion of proinflammatory cytokines such as tumor necrosis factor, interleukin (IL) 1a, and IL-8. Moreover, these agents increase the presence of metalloproteinases (MMP) (MMP-1, MMP-2, and MMP-9), inducing collagen degradation and, as a result, photoaging.24
Ultrafine particles are those that measure less than 100 nm in diameter and have greater harmful potential for health than PM. These include carbon black and PAHs, both associated with skin cancer.25 There is evidence of an increase in the incidence of melanoma, but not NMSC, in workers exposed to carbon black.26
Nevertheless, pollution can also contribute to a reduction in the amount of UV radiation that reaches the surface of the earth. Thus, decreases of up to 50% are seen in UVB radiation levels on days of high pollution. However, UVA radiation is not attenuated by pollution, and so the ratio of UVB/UVA that reaches the earth’s surface may be impacted, and the effects of this change are as yet unknown.19
Moreover, all agents that we are exposed to can interract to produce synergistic or antagonistic effects. UV radiation in combination with PAHs increases photodamage to the skin,27 while UVA radiation increases the carcinogenic activity of benzopyrenes.28 Furthermore, the microbiota of the skin, and micrococci in particular, may degrade benzopyrenes, and as such they would constitute an innate defence mechanism against PAHs.29
The most relevant epidemiological study to investigate the relationship between pollution and skin cancer was conducted in Saxony; the study assessed the incidence of skin cancer between 2010 and 2014 in approximately 2 million individuals and found a relationship with existing pollution.30 In particular, the authors found an association between PM10 (10 µg/m3) and NO2 and an increase in the relative risk of NMSC of 52% and 7% to 24%, respectively. In contrast, in this study, a protective effect of green areas was found, as an increase in 10% of such areas reduced the relative risk of NMSC by 16%. One limitation of this study was that socioeconomic level and smoking habit were not taken into consideration.30
PesticidesAn increased exposure to pesticides has been shown to increase the risk of developing melanoma (odds ratio [OR], 2.1; 95% confidence interval [CI], 1-6.9), especially when exposed in an occupational setting (OR, 4.23; 95% CI, 1.94-6.31).31
The AGRICAN Cohort study, conducted in a cohort of 11 000 French agricultural workers followed for up to 6 years, reported a significantly higher incidence of multiple myeloma in men and melanoma in women who had been exposed to pesticides.32
Moreover, a synergistic effect between exposure to pesticides and UV radiation has been demonstrated, increasing the risk of melanoma with an OR increasing from 2.6 (95% CI, 1.2-5.6) to 4.7 (95% CI, 1.3-17).33
Genetics has a modulating effect on these risk factors. Certain polymorphisms in the glutathione S-transferase (GST) gene, along with exposure to pesticides, has been associated with a greater risk of cutaneous melanoma. Likewise, the unmutated GSTM1 gene is a risk modifier for cutaneous melanoma in patients exposed to pesticides with an OR of 2.8 (95% CI, 1.1-7.1).34
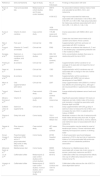
Smoking HabitEpidemiological studies published on the risk of skin cancer in smokers have yielded contradictory results (Table 1).35–40
Epidemiological Studies of the Association Between Skin Cancer and Smoking Habit.
| Reference | Type of Study | Number of Individuals | Results of Association With Smoking Habit |
|---|---|---|---|
| Thompson et al.35 | Metaanalysis (6 cohort studies and 8 case-control studies) | 738661 | Increased risk of head and neck melanoma |
| Song et al.36 | Metaanalyses and cohort study | 145709 | No association with risk of SCC was found |
| An inverse relationship with the onset of melanoma and BCC was found | |||
| Jones et al.37 | Cohort study | 4231 | Correlation with the appearance of lymphatic metastasis, independently of the correlation with tumor thickness and ulceration |
| Dusingize et al.38 | Cohort study | 38697 | Lower risk of melanoma in former smokers compared with nonsmokers or current smokers |
| Nagore et al.39 | Case-control study | 160 patients with melanoma, aged over 60 years | Smoking habit is an independent risk factor in individuals aged over 60 years |
| 318 controls | |||
| Espinosa et al.40 | Cohort study | 1200 patients with melanoma | No association was found between smoking more than 20 packet-years as a risk factor for developing NMSC in patients with a history of melanoma |
In terms of genetics, some single nucleotide polymorphisms in the CHRNA5-A3-B4 gene have been associated with smoking and play a role in the development of melanoma.41 Furthermore, 2 polymorphisms in the vitamin D receptor gene (BsmI-rs1544410 and FokI-rs2228570) have been linked to susceptibility to obesity, duration of smoking habit, and severity of melanoma.42
Experimental animal studies have shown that exposure to UV radiation and cigarette smoke has a synergistic effect for inducing SCC in mice.43
Pathogenesis is established because cigarette smoke increases epidermal proliferation and induces ROS that oxidize DNA in fibroblasts. Furthermore, the affected fibroblasts induce the secretion of IL1α, IL-6, IL-8, basic fibroblast growth factor (FGF), monocyte chemotactic protein (MCP) 1, and insulin growth factor type 4 (IGFBP4), which stimulate the proliferation of keratinocytes, thereby favoring carcinogenesis in skin thorugh regulation of cell proliferation. Acute oxidative stress compromises the DNA repair systems, increasing the repair intervals and increases damage in the DNA in groups of smokers.44,45
Finally, it has been observed that smoking is a modifiable risk factor for the onset of other extracutaneous neoplasms in patients with a personal history of NMSC or melanoma.46
DietThe effect of diet on different dermatoses and neoplasms is a topic subject to debate.47,48
With respect to vitamin intake, studies have found an inverse relationship between serum levels of vitamin A (retinol) and its derivative β-carotene with developing NMSC,49,50 whereas others have found the opposite.51,52 Clinical trials have assessed the effect of oral retinol supplements on the prevention of skin cancer and, although most have not found a protective effect,53–55 it appears that the risk of SCC is reduced in patients with a moderate initial risk.56
Loss of the vitamin D (VD) receptor increases susceptibility to UV-induced skin tumorigenesis in mice.57 However, studies in humans support an inverse effect of VD levels on risk of NMSC.58 A confounding factor is sun exposure, which is positively correlated with both serum VD levels and skin cancer. The role of VD in epidemiological studies is not clear, although several pathological and molecular studies have shown a correlation between VD deficit and progression and prognosis of melanoma.59 Recently, in a study of 204 invasive melanomas, the role of VD was assessed. A significant association was found between VD levels on diagnosis and site, mitotic index of the tumor, and ulceration, while a borderline association with Breslow depth and body mass index was also found, suggesting a role for VD levels in determining the aggressiveness of melanoma.60
Selenium, a micromineral antioxidant, was postulated as a supplement that could decrease the effects of UVB radiation on DNA; however, in a clinical trial that administered an oral daily selenium supplement (200 μg) to patients with a history of 2 or more BCC or one or more SCC, paradoxically, the risk of SCC increased.61
In a cohort study, intake of folic acid in diet was associated with a moderate increase in the risk of cutaneous melanoma.62 However, the scientific evidence is still insufficient and other factors related to folic acid intake in diet that could explain this association need to be considered.62
Contaminants in diet are, however, a serious problem, particularly when they involve the water supply. Thus, it has been demonstrated that certain water contaminants, such as arsenic and polychlorinated biphenyls, may cause skin cancer. The WHO has established that there is a risk of skin cancer when the arsenic concentration in water exceeds 10 μg/L.63 The issue was highlighted by the observation that the risk for developing keratinocytic tumors was 1.5-fold greater in any rice consumers compared with nonconsumers (95% CI, 1.1-2.0).64 The reason why rice has the arsenic content it does is because it is a crop that requires abundant water and this, depending on the geographic area, may contain notable quantities of arsenic. Thus, a clear association has been documented between BCC and SQQ with arsenic concentrations < 100ug/L in South Asia, China, Taiwan, Mexico, eastern Europe, and the United States.63
Furthermore, the effect of arsenic is modified by genetic susceptibility, as occurs in polymorphisms of the gene encoding the arsenic methyltransferase gene; these polymorphisms decrease the capacity to metabolize arsenic.65
Polychlorinated biphenyls (PCPs) are extensively distributed in the environment, whether through direct dumping by industries that use them or through combustion and dumping in rivers and marine waters of contaminated waste. Fatty fish can contain significant quantities of polychlorinated biphenyls, which impact melanogenesis and facilitate melanoma progression. In contrast, these fish are also rich in σ-3 polyunsaturated fatty acids, which have an antineoplastic action on melanoma. A cohort study found a hazard ratio (HR) of 4 (95% CI, 1.2-1.3, P = .02) associated with PCBs, whereas for the relationship with eicosapentaenoic/docosahexaenoic acid intake, the HR was 0.2 (95% CI, 0.1-0.8, P = .003). Therefore, more studies are needed to really demonstrate whether consumption of this fish has a protective effect or may increase the risk of melanoma.66
The association of Mediterranean diet with skin cancer has also been studied. In French women, closer adherence to this diet was associated with a reduction in the overall risk of developing skin cancer, mainly through an inverse association with BCC incidence and a decrease in the risk of melanoma. These results should also be confirmed in men and possible biases assessed, but the results are sufficiently promising to be cited as an additional benefit when encouraging the Mediterranean diet in our patients.67
Coffee and caffeine consumption is inversely and proportionally related to the risk of melanoma in several studies. Specifically, consumption of a cup of coffee a day decreased the risk of melanoma by 3%.68 In contrast, a metaanalysis found a dose-dependent relationship between caffeinated coffee consumption and incidence of BCC.69
Finally, dietary restriction alters both diurnal sensitivity to DNA damage caused by UVB radiation and expression of the XPA gene, which is essential for DNA repair, demonstrating the link between circadian rhythm, food intake, and skin health.70 All these studies conducted in humans that link diet with risk or not of developing skin cancer are summarized in Table 2.48,58,60–64,66–69
Epidemiological Studies of the Association Between Skin Cancer and Diet and Nutrients.
| Reference | Diet and Nutrients | Type of Study | No. of Individuals | Findings of Association with Diet |
|---|---|---|---|---|
| Ruan et al.48 | Total and saturated fats | Metaanalysis (3 cohort studies and 9 case-control studies) | 30915 BCC | No association between dietary intake of total and saturated fat and BCC, SCC, and melanoma |
| 4106 SCC | High monounsaturated fat intake was associated with a decrease in risk of BCC (RR, 0.90; 95% CI, 0.85–0.96). High polyunsaturated fat intake was positively associated with risk of SCC (RR, 1.19, 95% CI, 1.06–1.33) | |||
| 1638 melanomas | ||||
| Kune et al.49 | Vitamin A and β-carotene | Case-control study | 176 (88 cases and 88 controls) | Inverse association with NMSC (BCC and SCC) |
| Cases also had lower serum levels of β-carotene and vitamin A than controls | ||||
| Wei et al.50 | Folic acid | Clinical trial | 958 | Supplementation with folic acid was not associated with BCC incidence |
| Fung et al.51 | Vitamins A, C and E and folates | Clinical trial | 5392 | There was no evidence that vitamins A, C and E and folates play a substantial protective role in the incidence of BCC |
| Karagas et al.52 | Selenium, α-tocopherol, β-carotene, and retinol | Case-control studies | 396 (132 cases and 264 controls) | At the concentrations studied, there was no relationship with the risk of SCC |
| Frieling et al.53 | β-carotene | Clinical trial | 22071 | Supplementation with β-carotene for an average of 12 years did not impact the risk of BCC or SCC |
| Green et al.54 | β-carotene | Clinical trial | 1383 | Supplementation with β-carotenes was not associated with a change in the risk of either SCC or BCC |
| Greenberg et al.55 | β-carotene | Clinical trial | 1805 | Supplementation with β-carotenes in individuals with NMSC did not reduce development of new NMSC |
| Moon et al.56 | Oral supplementation with retinol | Clinical trial | 2297 | Daily supplementation with 25000 IU of retinol was effective for preventing SCC, although it did not prevent BCC in patients with moderate risk |
| Tang et al.58 | Vitamin D | Case-control study | 178 cases 930 controls | Inverse relationship between serum levels and NMSC |
| Moreno-Arrones et al.60 | Vitamin D | Longitudinal study | 204 melanomas | Significant association between VD levels on diagnosis and site, mitotic index of the tumor, and ulceration; a borderline association with Breslow depth and BMI |
| Duffield-Lillico et al.61 | Selenium supplements | Clinical trial | 1312 | Daily supplementation with 200 μg daily of selenium does not reduce the risk of BCC and increases the risk of SCC in patients with a history of NMSC |
| Dhana et al.62 | Dietry folic acid | Cohort study | 75311 women and 48523 men | Moderate increase in the risk of melanoma with folate intake derived solely from diet. A higher intake of vitamins B6 and B12, choline, betaine, and methionine was not associated with risk of melanoma |
| Gossai et al.64 | Rice (arsenic content) | Case-control study | 487 cases 462 controls | Rice consumption may be related to onset of keratinocytic tumors, in those individuals with relatively low exposure to arsenic in drinking water |
| Donat-Vargas C et al.66 | Polychlorinated biphenyls (PCB) and σ-3 polyunsaturated fatty acids (in fatty fishes in diet) | Cohort study | 20785 Swedish women | A direct association was found between exposure to PCB in diet and risk of melanoma. Intake of σ-3 polyunsaturated fatty acids was shown to have a substantial protective association |
| Mahamat-Saleh et al.67 | Mediterranean diet | Cohort study | 98995 French women | Adherence to a Mediterranean diet is associated with a lower risk of skin cancer in women, particularly melanoma and BCC |
| Liu et al.68 | Caffeinated coffee | Metaanalysis (2 case-control studies and 5 cohort studies) | 851672 | Caffeinated coffee has chemopreventive effects. One cup of coffee daily reduces the risk of melanoma by 3% |
| Vasegui et al.69 | Caffeinated coffee | Metaanalysis (2 case-control studies, 4 cohort studies, and 1 longitudinal study) | 320370 controls and 104770 cases | Caffeinated coffee reduces the risk of BCC in dose-dependent fashion |
Numerous epidemiological studies have examined the relationship between physical activity and risk of NMSC and melanoma. Athletes who practice sports outdoors receive high doses of UV radiation due to the long hours of training and competition with high exposure to sunlight, and this effect increases in alpine sports due to the increase in UV radiation at altitude and reflection from surfaces coated with snow and ice.71 Thus, extreme exposure to UV radiation in outdoor sports such as skiing, mountain climbing, or cycling or triathlon has been documented in a series of dosing studies.72–75
Extensive epidemiological studies show that recreational activities such as sunbathing on the beach or exposure during aquatic sports are associated with a greater risk of BCC, whereas skiing has been shown to have a greater risk of SCC.76–78 The incidence of BCC and SCC has been calculated in different groups of sports enthusiasts, finding a greater risk of tumors in marathon runners, an increased incidence of BCC in surfers, and an increased prevalence of actinic keratosis in rock climbers. However, there are few studies, the sample sizes are small, and they do not include former sports player or retired athletes.76,77
A metaanalysis that included 13949 patients who had recently been diagnosed with melanoma found that in cohort studies, there is a greater risk of melanoma in patients with high physical activity (relative risk = 1.3; 95% CI = 1.2-1.4); however, this relationship was not shown in case-control studies, probably because of confounding factors such as exposure to sunlight and phototype.79
Some risk factors for cutaneous melanoma, such as number of melanocytic nevi and solar lentigines, are more frequent in individuals who practice sport outdoors. In addition to substantial exposure to sunlight, exercise-induced immunosuppression could increase the risk of NMSC and cutaneous melanoma in athletes.78,80
However, experimental studies suggest that exercise may have protective effects for skin cancer. In PTEN knockout mice, exercise prevents hepatocellular carcinoma and skin cancer induced by tetradecanoylphorbol-13-acetate (TPA) in an experimental animal model.81 Exercise stimulates secretion of circulating insulin growth factor (IGF-1) and the corresponding signal in TPA mice, reducing the risk of skin cancer. Furthermore, exercise activates p53, which increases p21, IGFBP-3, and PTEN, in turn inducing down-regulation of the IGF-1 pathway, contributing to prevention of skin cancer.82–84
In summary, exercise may have a protective effect on skin cancer, but not sufficient to compensate for the risk derived from excessive exposure UV radiation associated with outdoor sports; probably the equilibrium is negatively influenced by inadequate photoprotection habits. Therefore, protective measures should be encouraged in those who practice outdoor sports. Such measure include encouraging athletes to avoid training at times of maximum risk, choosing appropriate clothing, and applying water and sweat-resistant sunscreen.85
Circadian RhythmDisruption of the circadian rhythm has been classified by the International Agency for Cancer Research as one of the most probable carcinogens based on the evidence from human and animal studies.86
There is an increase in the standardized HR for melanoma in pilots (2.2 [95% CI, 1.7-2.9]) and also in cabin crew in aircraft of 2.1 (95% CI, 1.7-2.9). Penetration of UVA radiation through the windows is a plausible factor for pilots, but not for the rest of the crew. It has yet to be resolved whether the underlying cause for this observation is cosmic radiation, lifestyles with more holidays with greater exposure to sunlight, or disruption of the circadian rhythm.87,88
Another group within the population who works shifts and has a higher incidence of melanoma are firemen; in this group, the results have been variable and the risk of melanoma is estimated to be 1.3 (95% CI, 1.1-1.6).89 Nevertheless, it should be remembered that this group is exposed to potential carcinogenic compounds generated in fires, such as PAHs, which have been linked to NMSC.21
In other shift workers such as nurses, those who usually worked night shifts have an incidence of skin cancer 14% lower than those who worked night shifts less regularly or never.90
The influence of disruption to circadian rhythm on carcinogenesis is attributed to different possible mechanisms. On one hand, lower secretion of melatonin reduces anticarcinogenic and antioxidant acitivty91; on the other, there is a circadian regulation induced by UV radiation of certain DNA repair mechanisms, as well as antioxidant cutaneous mechanisms.92 However, the association with greater or lower incidence of skin cancer is not clear due to confounding factors, such as decreased exposure to UV radiation due to working night shifts.
Socioeconomic StatusThe impact of social and economic levels on cancer survival have been studied. Economic inequalities in countries with limited healthcare access are responsible for worse melanoma prognosis because the tumor is diagnosed in more advanced stages.93 Thus, a strong inverse correlation has been demonstrated between total healthcare spending per head and the ratio of melanoma incidence to the corresponding mortality (r = -0.76, P< .05).94
In Germany, a study was conducted in 70 million inhabitants, finding a direct correlation between a larger number of admissions and a lower level of education with a higher prevalence of melanoma and NMSC.95
A multicenter study in 5 European countries (France, Germany, Portugal, Italy, and Sweden) found that there is a certain increment in the risk of skin cancer associated with greater socioeconomic level in middle-aged patients, without any differences in older individuals.96
Microorganisms and MicrobiotaCertain microorganisms are known to play a role in some skin cancers, in particular, Merkel cell polyomavirus leading to Merkel cell carcinoma and human papillomaviurs.97 In the case of Merkel cell polyomavirus, in a study of water analysis in Mediterranean countries, this entity was found in 75% of residual waters and in 29% and 18% of samples taken from rivers and the sea, respectively. The study affirms that water treatment and UV radiation eliminate between 2.22 and 4.52 log10 viral concentrations, but global warming and limited rainfall could increase the presence of this virus and other water-born viruses.98
It is known that Propionibacterium acnes, one of the bacteria in our skin microbiota, decreases synthesis of porphyrins on exposure to UVB radiation.99 UV radiation inhibits growth of Malassezia furfur, a yeast in the microbiota that synthesizes pityriacitrin, a substance with a photoprotective effect.100 Furthermore, a relationship has been reported between development of melanoma and microorganisms of the Fusobacterium and Trueperella genera.101 On the other hand, skin colonization of nude mice with a strain of Staphylococcus epidermidis from human microbiota that synthesizes 6-N-hydroxyaminopurine exercises a preventive role in the development of skin tumors in a model of photocarcinogenesis with UVB; in contrast, colonization by a strain of the same bacterium that does not produce 6-N-hydroxyaminopurine did not have any preventive effect.29 This could indicate that certain bacteria in the microbiota could lead to a protective effect or induce cancer when combined with UV radiation or sunlight.
But it is not just skin microbiota that might impact skin cancer. Changes in the intestinal microbiota have been observed to bear a relationship with different responses to immunotherapy, such that a greater diversity in intestinal microbiota and a greater growth of the Ruminococcaceae family favors response to immunotherapy with anti-PD-1 drugs.102,103
ConclusionThis literature review shows that not only known factors such as UV and electromagnetic radiation but also factors such as environmental pollution, including air pollution, smoking, and water contamination, may have an impact on skin health and cancer genesis. Some data are still contradictory or inconclusive, and individual susceptibility, determined by the genome itself, along with perhaps other genes that cohabit with us, in the microbiome, should be taken into account.
Please cite this article as: González S, Parrado C, Gilaberte Y. La influencia del exposoma en el cáncer de piel. Actas Dermosifiliogr. 2020;111:460–470.