Pyoderma gangrenosum is a rare neutrophilic skin disease that may be idiopathic or associated with systemic disease such as inflammatory bowel disease, arthritis, paraproteinemia, or blood cancers. While it is true that topical or intralesional therapies can be used in patients with isolated lesions that have little impact on quality of life, in most cases, systemic immunosuppressant treatment is required. Corticosteroids and cyclosporin are used as the first line in systemic treatments. Other reported alternatives, although not approved for this indication, include mycophenolate mofetil, anti-TNF, intravenous immunoglobulins, interleukin (IL) 1 antagonists, and ustekinumab (anti-IL-12/23).1
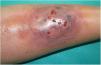
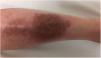
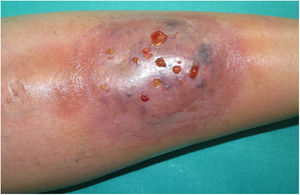
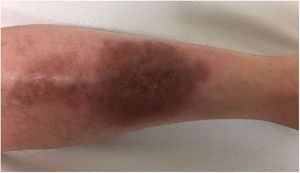
A 33-year-old woman diagnosed with ulcerative colitis since the age of 17 years had presented multiple pyoderma gangrenosum lesions on the lower limbs throughout the course of the disease. The patient had been treated with multiple therapies that, in chronological order, included 6-mercaptopurine, cyclosporin at 3-5mg/kg/d for 5 months, intravenous infliximab at 5mg/kg every 4 weeks, subcutaneous adalimumab at 40mg/wk, plasmapheresis every 15 days, tacrolimus oral at 8mg/d, intravenous vedolizumab at 300mg every 4 weeks, and subtotal colectomy. Cyclosporin, infliximab, adalimumab, and tacrolimus oral had been administered with the above treatments for the indication of pyoderma gangrenosum and topical treatment was associated with intralesional triamcinolone acetonide, and topical tacrolimus and clobetasol propionate. All previous treatments were associated with prednisone at doses of up to 50mg/d. At one point in the course of the disease, the patient presented left pretibial pyoderma gangrenosum measuring 11×7cm. The lesion, which had appeared 5 months earlier, was difficult to manage, corticosteroid-dependent (it worsened when prednisone was reduced to below 20mg/d), and had a considerable impact on quality of life (Fig. 1). At the time, the patient was undergoing treatment with intravenous vedolizumab at a dosage of 300mg every 4 weeks, prednisone at 50mg/d, and topical corticosteroids. In light of the refractory nature of the disease and as multiple lines of treatment had been exhausted, it was decided to instate treatment with subcutaneous ustekinumab 90mg (week 0, 4, 10, and every 8 weeks thereafter) associated with cyclosporin at a dose of 3mg/kg/d, with an excellent response of the pyoderma gangrenosum, which resolved completely in 12 weeks, making it possible to reduce the dosage of prednisone to 5mg/d (Fig. 2). After 10 months of follow-up, the patient has maintained treatment with ustekinumab every 8 weeks with very good results; this has made it possible to withdraw the cyclosporin and reduce the prednisone to 2.5mg every 2 days.
In 2011, Guenova et al reported the first case of pyoderma gangrenosum successfully treated with ustekinumab.2 Those authors suggested that IL-23 is implicated in the pathogenesis of immunologic diseases such as psoriasis and inflammatory bowel disease; they therefore studied the expression of this interleukin in the patient's lesion biopsy and observed overexpression of IL-23 compared to biopsies of healthy skin. Ustekinumab was therefore proposed as a targeted therapy for treatment of the pyoderma gangrenosum.2 Since then, 5 more cases have been reported in the literature with good response to ustekinumab at doses ranging from 45-90mg every 8 weeks to 135mg every 6 weeks (Table 1).2–7
Summary of Cases of Pyoderma Gangrenosum Treated With Ustekinumab Reported in the Literature.
| Author | Sex/Age, y | Associated Disease | Previous Treatment | Ustekinumab Dosage | Response |
|---|---|---|---|---|---|
| Guenova et al.2 (2011) | Female/37 | None | Prednisolone and tacrolimus topical | 45mg/8 weeksAssociated with tacrolimus topical | Complete response reached after 14 weeks |
| Fahmy et al.3 (2012) | Female/34 | Ulcerative colitis | Corticosteroids, azathioprine, infliximab, colectomy, adalimumab, and tacrolimus oral | 90mg/8 weeks | Complete response reached after 10 weeks |
| Goldminz et al.4 (2012) | Male/37 | None | Topical, intralesional, and systemic corticosteroids, cyclosporin, azathioprine, methotrexate, adalimumab, infliximab, and golimumab | 90mg/8 weeksAssociated with dapsone 100mg/d and prednisone | Complete response reached after 22 weeks |
| Cosgarea et al.5 (2016) | Female/71 | Renal cell carcinoma | Prednisone and cyclosporin | Not reported | Complete response reached 12 weeks after reinstatement of ustekinumab following surgery for renal cell carcinoma (no response prior to surgery) |
| Greb et al.6 (2016) | Male/50 | None | Prednisone, cyclosporin, adalimumab, infliximab, and golimumab | 90mg/8 weeks to 135mg/6 weeksAssociated with dapsone 100mg/d and prednisone | Response reached after 16 weeks following higher dosage of ustekinumab |
| Benzaquen et al.7 (2017) | Female/56 | Psoriasis treated with adalimumab (induced by anti-TNF) | None | 45mg. Interval not specified | Complete response after 3 doses |
In conclusion, we present a new case of pyoderma gangrenosum associated with ulcerative colitis, with good response to ustekinumab together with cyclosporin and oral corticosteroids following a lack of response to the 2 previous treatments, tacrolimus oral and anti-TNF (infliximab and adalimumab), administered together. We propose this alternative in difficult-to-manage cases of pyoderma gangrenosum that are refractory to other treatments. Further studies are required to determine the involvement of IL-23 in the pathogenesis of this disease.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Piqueras-García J, Sahuquillo-Torralba AJ, Torres-Navarro I, Botella-Estrada R. Pioderma gangrenoso asociado a colitis ulcerosa con buena respuesta a ustekinumab Eczema y urticaria en Portugal. 2019;110:776–778.