Nonalcoholic fatty liver disease (NAFLD) is the most prevalent liver condition in the West. The prevalence and severity of NAFLD is higher and the prognosis worse in patients with psoriasis. The pathogenic link between psoriasis and NAFLD is chronic inflammation and peripheral insulin resistance, a common finding in diseases associated with psoriasis. NAFLD should therefore be ruled out during the initial evaluation of patients with psoriasis, in particular if they show signs of metabolic syndrome and require systemic treatment. Concomitant psoriasis and NAFLD and the likelihood of synergy between them place limitations on general recommendations and treatment for these patients given the potential for liver toxicity. As hepatotoxic risk is associated with some of the conventional drugs used in this setting (e.g., acitretin, methotrexate, and ciclosporin), patients prescribed these treatments should be monitored as appropriate. Anti-tumor necrosis factor agents hold the promise of potential benefits based on their effects on the inflammatory process and improving peripheral insulin resistance. However, cases of liver toxicity have also been reported in relation to these biologics. No evidence has emerged to suggest that anti-p40 or anti-interleukin 17 agents provide benefits or have adverse effects.
El hígado graso no alcohólico es la principal causa de enfermedad hepática en nuestro medio. Los pacientes con psoriasis presentan mayor prevalencia y gravedad y peor pronóstico de esta hepatopatía. El vínculo patogénico entre ambas es el estado de inflamación crónica y la resistencia periférica a la insulina, habitual en las comorbilidades asociadas a la psoriasis. Por este motivo, en la evaluación de los pacientes con psoriasis, en particular si existen componentes del síndrome metabólico y se requiere tratamiento sistémico, se recomienda descartar esta posibilidad. La coexistencia de psoriasis e hígado graso no alcohólico, con probable sinergia entre ambos, condiciona las medidas generales que deben recomendarse en estos pacientes y también la estrategia terapéutica, por la potencial hepatotoxicidad de algunos de ellos. En este sentido, algunos de los fármacos convencionales habituales como acitretino, metotrexato o ciclosporina presentan potenciales efectos hepatotóxicos cuya repercusión en cada paciente debe evaluarse de forma individualizada. Los fármacos anti-TNF podrían tener efectos beneficiosos fundamentados en el buen control del proceso inflamatorio y de una mejoría de la resistencia periférica a la insulina. Sin embargo, se han descrito casos de hepatotoxicidad en algunos pacientes. No existe evidencia de efectos beneficiosos o perjudiciales de los fármacos anti p40 o anti IL-17.
Nonalcoholic fatty liver disease (NAFLD) is a process in which fat is deposited in the liver in the absence of significant alcohol consumption or the use of drugs—such as steroids, methotrexate (MTX), tamoxifen, or amiodarone—that facilitate steatosis.1 Certain hereditary conditions such as Wilson disease, abetalipoproteinemia, Wolman disease, and cholesteryl ester storage disease are also incompatible with a diagnosis of NAFLD.1 Once these conditions are excluded, nearly all patients diagnosed with NAFLD share the characteristics associated with metabolic risk, such as obesity, type 2 diabetes mellitus, hypertension, and dyslipidemia. NAFLD and metabolic syndrome share the pathophysiologic mechanism of insulin resistance.2
Two histologic forms of the disease can be distinguished: one is steatosis, in which fat has been deposited but liver cells are not yet damaged, and the other is steatohepatitis, which is characterized by liver inflammation that can eventually lead to fibrosis. Mortality is higher in patients with NAFLD than in the general population, regardless of which of these histologic variants is present; the cause of death is usually cardiovascular disease.3 Because steatohepatitis is an inflammatory condition, the liver is threatened, and 15% to 25% of patients will have progressed to cirrhosis within 10 years. Once cirrhosis develops, complications (liver failure, esophageal varices, and liver cancer) will lead to death in 25% of affected patients within 5 years.4 Why fat deposition causes inflammation and fibrosis in some patients but not others is poorly understood, although genetic and environmental factors are probably both relevant.5
NAFLD is important in the West because it is currently the most prevalent liver disorder. Approximately 20% of adults in the general population have NAFLD, and among them about 20% have the aggressive form, steatohepatitis.6 The prevalence is much higher among adults with metabolic syndrome, where 38% of overweight persons (with a body mass index [BMI] between 25 and 30) have NAFLD. The prevalence rates in obese (BMI, 30–40) and severely obese (BMI, 40–45) persons are 46% and 65%, respectively. Finally, some 90% of morbidly obese patients (BMI>45) have this condition, and 5% of them have cirrhosis. Among diabetics, the prevalence is 69%, and among patients with elevated triglyceride levels, 50%.7 A sign of the seriousness of this situation is that nonalcoholic steatohepatitis is currently the second most common cause of liver transplantation in the United States, and it is expected to become the first cause in the coming years.8
In spite of these statistics, NAFLD is clearly underdiagnosed because there are no signs or symptoms that arouse suspicion except when the disease becomes advanced and also because serum biochemical markers show only slight, nonspecific changes; results may even stay within the normal limits in 15% to 39% of patients.9,10
A diagnosis of NAFLD requires evidence of steatosis by liver biopsy or imaging and the exclusion of alcohol consumption or other general causes of liver disease or conditions that lead to steatosis.1 The first criterion—evidence of steatosis—is critical. The list of causes of liver disease are well established11 while the alternative causes of steatosis are very rare and, in principle, relatively easy to identify. A liver biopsy is the gold standard, but it is invasive, has associated risk of complications and death, and is costly.12 Furthermore, it is not reasonable or even feasible to biopsy 20% of the population. Interest in finding a noninvasive approach to diagnosis is therefore growing. A fatty liver is usually detected through imaging (ultrasound, computed tomography scans, or nuclear magnetic resonance); the sensitivity and specificity of images are 60% and 94%, respectively.13,14 Ultrasound is usually chosen because it is innocuous and widely available.1 However, neither liver enzymes nor images can distinguish between steatosis and steatohepatitis. Nor can they establish or rule out the presence of fibrosis. Various scoring systems using the results of serum tests have therefore been developed. The most widely accepted is the NAFLD fibrosis score,15 which is calculated according to a formula (http://nafldscore.com) based on easy-to-obtain measurements: BMI; age; platelet count; and serum albumin, aspartate transaminase (AST), and alanine transaminase (ALT) (Table 1). Moreover, this score is highly sensitive (67%) and specific (60%) for excluding (67%) or detecting (97%) the presence of advanced fibrosis using an area under the receiver operating characteristic curve of 0.85 as the cutoff. The severity of liver damage can also potentially be assessed by measuring liver stiffness indirectly with elastography. The usefulness of this approach would mainly be to identify patients at low risk of advanced fibrosis or cirrhosis given the test's lack of precision when disease is advanced.16
Formula for the NAFLD Fibrosis Score and Risk Interpretation a
| NAFLD Fibrosis Score=–1.675+0.037×age in y+0.094×BMI (kg/m2)+1.13×glucose intolerance or diabetes (yes=1, no=0)+0.99×AST/ALT ratio–0.013×platelet count (×109/L)–0.66×albumin (g/dL) |
| Calculator available from: http://nafldscore.com |
| A score <–1.455 predicts low risk for advanced fibrosis. |
| A score >0.676 predicts advanced fibrosis. |
| Risk is indeterminate if the score falls between –1.455 and 0.676. |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; NAFLD, nonalcoholic fatty liver disease.
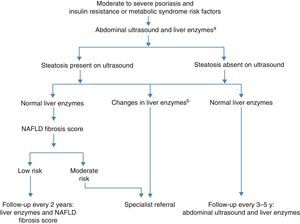
The European Association for the Study of the Liver, the European Association for the Study of Diabetes, and the European Association for the Study of Obesity have recently published new joint guidelines for managing NAFLD.17 They recommend that all patients with insulin resistance or risk factors for metabolic syndrome be screened for NAFLD by ultrasound and liver biochemistry. Any increase in AST, ALT, and/or γ-glutamyltransferase are relevant, regardless of magnitude. The screening algorithm is shown in Fig. 1.17
Nonalcoholic fatty liver disease (NAFLD) in patients with psoriasis. Adapted from clinical guidelines.17
a Aspartate transaminase (ASP), alanine transaminase (ALT), and γ-glutamyltransferase (GGT).
bAny increase in ASP, ALT, and/or GGT.
At this time there is no specific treatment for NAFLD available. Weight loss of at least 7% to 10% and exercise are associated with histologic improvement,18 but they are difficult to achieve and particularly difficult to maintain long-term. Alcohol, saturated fats, and fructose-sweetened beverages should be avoided.19 Coffee consumption, on the other hand, can be recommended.20 Several drugs (metformin, pioglitazone, eicosapentenoic acid, statins, and vitamin E) have been tried, but there is a lack of sufficient evidence on which to base a strong recommendation.1,6,21–24
NAFLD and PsoriasisThe first observational studies showing an association between NAFLD and psoriasis began to appear during the last 10 years,25,26 and the association has since been confirmed.27,28 Gisondietal.25 saw a higher rate of ultrasound-diagnosed NAFLD (47%) in a series of 130 consecutive patients with psoriasis than in 260 healthy controls (28%) matched for age, sex, and BMI. The psoriasis patients were also more likely to have metabolic syndrome and higher C-reactive protein levels; those who also had NAFLD had higher interleukin (IL) 6 and lower adiponectin levels than those without the disease. Abedinietal.28 reported finding NAFLD in 65.6% of patients with psoriasis (vs 35% of healthy controls), and vanderVoortetal.27 found that 46.2% of psoriatic patients and 33.3% of controls had NAFLD in a population-based series of 2292 persons over the age of 55 years in Rotterdam. These findings may be attributable to the association between psoriasis and metabolic syndrome, given that the conditions defining the latter are those that lead to NAFLD.29 Mieleetal.26 studied a cohort of psoriasis patients with and without NAFLD, finding that NAFLD was also associated with psoriatic arthritis. Robertsetal.30 reported finding a relationship between greater severity of psoriasis and NAFLD. Specifically, they saw steatohepatitis in 22% of the patients with both psoriasis and NAFLD who underwent liver biopsy. The NAFLD patients with steatohepatitis were also more likely to be obese and have higher insulin and transaminase levels; they also found marked differences in risk for NAFLD according to ethnic origin. Similarly, vanderVoortetal.27 found that patients of Latin American origin carried greater risk (83%), followed by Caucasians (39%), and finally African Americans (33%).
Clinical Suspicion and Screening for NAFLD in DermatologyA high index of suspicion concerning NAFLD is needed in dermatology because most cases encountered will be asymptomatic. The dermatologist should above all be aware of the high prevalence in patients with psoriasis. It is also important to note signs of the components of metabolic syndrome: diabetes mellitus, obesity, dyslipidemia, and hypertension. Abnormal liver enzymes may be difficult to discern because elevations are usually slight, at less than 2- to 3-fold the normal values and with an ALT/AST ratio greater than 1; alkaline phosphatase and γ-glutamyltransferase are occasionally elevated.31 Liver enzymes can be normal in 15% to 30% or more of patients with NAFLD,10 even though 35% of such patients have advanced fibrosis or cirrhosis. Given this situation, in the presence of the most prevalent indicator of risk for NAFLD—namely components of metabolic syndrome—ultrasound imaging to rule out liver disease is indicated even when biochemistry is normal.17 At this time, however, the clinical algorithm recommended by most medical associations specializing in NAFLD (Fig. 1), does not recognize the need for imaging to evaluate patients with psoriasis in the absence of liver enzyme abnormalities.
Once NAFLD has been confirmed, the dermatologist must cooperate in prescribing the necessary dietary measures and adjusting drug dosages if transaminase levels are elevated.
The absence of liver damage according to the initial assessment does not exclude its development over the coming years. Therefore, patients will have to be reassessed if analyses reveal signs of damage or if metabolic syndrome develops.17
Pathogenesis of NAFLD in PsoriasisThis review does not attempt to delve deeply into the pathogenesis of NAFLD, but it is useful to be aware of the two-hit hypothesis.32 In this theory, the first phase would be marked by the abnormal accumulation of triglycerides in liver cells, a development in which insulin resistance plays a large role. In the second phase, the steatotic liver appears to become susceptible to injury induced by several players and events involving various adipokines and oxidative stress in the endoplasmic reticulum, as well as mitochondrial dysfunction and liver apoptosis. These events favor the transition from simple steatosis to steatohepatitis. Besides the involvement of insulin resistance in both these phases of progression toward NAFLD—as well as in the induction and activation of profibrotic cytokines—other chronic inflammatory processes that might be present, such as psoriasis, can contribute proinflammatory cytokines.33 Thus, the pathogenic relationship between NAFLD and psoriasis is complex and probably multifactorial, although it is presumably related to a state of chronic underlying inflammation.
NAFLD and psoriasis are probably linked partly because some of the conditions that often accompany psoriasis are also the ones that cause NAFLD. Among them, obesity stands out. The epidemiologic association between obesity and psoriasis is clear, particularly in severe psoriasis, and the influences probably operate in both directions, such that obesity favors psoriasis and vice versa.34 The prevalence of NAFLD is also clearly higher in obese patients, especially those with excess abdominal fat, which is more metabolically active than subcutaneous fat.35 Obesity is associated with risk for developing insulin resistance, the main factor in the development of NAFLD.36 Excessive fat deposition leads to an imbalance between pro- and anti-inflammatory cytokines, favoring the progression of liver disease. When adipose tissue secretes an abundance of adipokines—tumor necrosis factor (TNF), IL-6, leptin, visfatin, or resistin—a proinflammatory state may develop in psoriatic skin as the result of the proliferation of keratinocytes, angiogenesis, the response of type 1T cells, or the expression of adhesion molecules. This state may also be expressed in NAFLD, contributing to insulin resistance or liver fibrosis. In addition, there is also a decrease in the concentration of adipokines that protect against inflammation—such as adiponectin, a promotor of skin anti-inflammatory cytokines that enhances insulin sensitivity.37
The involvement of IL-17 in both psoriasis and NAFLD is interesting. T cells in adipose tissue synthesize IL-17, which is able to regulate adipogenesis and glucose metabolism. Type 17T helper cells and IL-17 could facilitate the progression from simple steatosis to steatohepatitis.38
Implications for the Management of Conventional Systemic TreatmentsThe high prevalence of NAFLD in psoriasis leads to therapeutic concerns because this chronic skin disease must be treated over decades.
The incidence of MTX-induced liver damage is known to be higher in psoriasis39 than in other chronic inflammatory diseases. A recent retrospective study saw, for example, that 58% of 710 patients on MTX for psoriasis had elevated transaminase levels.40 In the light of our current understanding, this finding may be relevant to the known association between NAFLD and psoriasis. Given the high rates of both NAFLD and steatohepatitis in this setting, we should rule out significant steatosis before starting MTX, at least in patients with signs of metabolic syndrome. In addition to ultrasound screening, two new tools, the FibroTest and the FibroScan (liver elastography) may be useful for assessing liver fibrosis.41
MTX toxicity presents with histologic findings and may therefore reflect pathogenic mechanisms that are different from those associated with NAFLD. However, the shared risk factors that aggravate both MTX toxicity and NAFLD—such as obesity, diabetes, or hyperlipidemia—are observed often in patients with severe psoriasis.42
Liver toxicity is also among the potential adverse effects of ciclosporin. The effect may be direct or it may be a byproduct of a deteriorating lipid profile.43 Acitretin can also raise triglyceride levels and has been associated with transaminase elevations in 1 out of 4 patients, although tissue damage following intermittent use of the drug has not been reported.44
Biologic Agents in Patients With Psoriasis and NAFLDTNF plays a central role in the inflammatory process in NAFLD, as in other chronic inflammatory diseases.45 In fact, TNF has been studied for its potential use as a diagnostic and prognostic tool in NAFLD.46 Given the pathogenic role of this cytokine, anti-TNF agents such as infliximab and adalimumab have also been tried in this setting47,48 because of improvements observed in liver and metabolic biomarkers after biologic therapy for other inflammatory diseases.49,50
Biologics have a better safety profile than the conventional treatments for NAFLD mentioned earlier. For example, when adalimumab was used for an average of 5 years in 32 patients with liver disease and psoriasis, no cases of liver disease progression or development of liver toxicity were observed.51
Biologic therapy could exercise regulatory effects on some adipocytokines. Shibataetal.52 reported an increase in adiponectin and IL-6 levels during treatment with infliximab, and adalimumab and etanercept may inhibit proinflammatory adipocytokines.53
Studies of the impact of anti-TNF agents on the lipid profile, a risk factor for NAFLD, have yielded inconsistent results. While some authors have not been able to demonstrate a significant effect, others have reported elevated triglycerides and lower high-density lipoprotein (HDL) cholesterol levels in patients with infliximab-treated psoriatic arthritis.54 Etanercept therapy, on the other hand, has favorably modulated the antioxident and anti-inflammatory properties of HDL cholesterol as well as modulated adipolipoprotein A1 and B levels, reducing lipid peroxidation.55
Marra et al.56 showed improved insulin sensitivity in patients treated with etanercept for psoriasis, an effect that could potentially have a favorable influence on the course of NAFLD.
It must be borne in mind that anti-TNF agents can occasionally induce autoimmune or drug-related hepatitis.57 The risk is very low, however. Only 20 cases of autoimmune hepatitis have ever been reported; most were mild cases associated with infliximab and responded quickly to steroid treatment.41 Anti-TNF agents have also been linked to weight gain in psoriasis patients, although the gains have generally been modest. However, long-term use has led to considerable gain in some patients, and this adverse effect potentially aggravates the obesity associated with severe psoriasis and NAFLD.58
High transaminase levels were detected in 6 patients in a series of 44 treated with ustekinumab for an average of 46.7 months.59 Five of the 6 patients developed hepatotoxicity related to other drugs, 3 developed fatty liver, and 3 were receiving other hepatotoxic drugs while taking ustekinumab. The authors concluded that ustekinumab-related toxicity was mild and uncommon. Hepatotoxicity is not described as a side effect detected in clinical trials of secukinumab60 (Table 2).
Main Findings of Studies on the Prevalence and Features of NAFLD in Psoriasis.
| Authors | Study Population | Psoriasis, n | No Psoriasis, n | Findings | Ethnicity |
|---|---|---|---|---|---|
| Roberts et al.30 | Patients with psoriasis or psoriatic arthritis | 103 | – | NAFLD prevalence, 47%. Steatohepatitis in 22% of patients with NAFLD. | Caucasians, Latin Americans, African Americans, Asians |
| Gisondi et al.25 | Hospitalized dermatology patients; controls, hospital staff | 130 | 260 | NAFLD prevalence higher in psoriasis patients (47%) than controls (28%) (P<.0001). Metabolic syndrome and elevated CRP levels more prevalent in patients with psoriasis and NAFLD (n=61), who also had higher mean (SD) PASI levels: 14.2 (12.6) vs 9.6 (7.4) for patients without NAFLD (n=69) (P<.01). | Not reported (Caucasians?) |
| Miele et al.26 | Patients with psoriasis; patients with NAFLD without psoriasis | 142 | 125 | 59.2% were diagnosed with NAFLD. Metabolic syndrome, obesity, dyslipidemia, elevated AST/ALT ratio, and psoriatic arthritis frequencies were higher in patients with both psoriasis and NAFLD. NAFLD patients with psoriasis had more severe liver disease than patients without psoriasis. | Not reported (Caucasians?) |
| Abedini et al.28 | Patients with psoriasis vs healthy controls | 123 | 123 | Prevalence of NAFLD was higher in psoriasis patients (65.6% vs 35% in controls) (P<.01, OR=3.53). Moderately severe NAFLD (grade 2) was more common than mild NAFLD (grade 1) (P<.01) in psoriasis patients. Higher rates of hypertension (16.5%), altered liver function (16.4%), and metabolic syndrome (46.6%) also found in patients with psoriasis and NAFLD. | Not reported (Caucasians?) |
| van der Voort et al.27 | Patients >55 years with NAFLD and psoriasis vs NAFLD patients without psoriasis | 118 | 2174 | 46.2% had NAFLD and psoriasis; 33%, only NAFLD. Psoriasis continued to be a predictor of NAFLD after controlling for alcohol consumption, ALT level, and presence of metabolic syndrome. | 95% Caucasian |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; PASI, Psoriasis Area and Severity Index.
NAFLD is the most frequent liver disorder in the West (Table 3). Patients with psoriasis have a higher prevalence of NAFLD, a higher rate of severe disease, and a worse prognosis. Dermatologists should routinely evaluate psoriasis patients for NAFLD. The pathogenic links between psoriasis and NAFLD are chronic inflammation and peripheral insulin resistance, which is a common finding in diseases associated with psoriasis. The dermatologist should be aware not only of the high prevalence of NAFLD in psoriasis but also of the possibility of steatohepatitis, especially when psoriasis is severe or when there are signs of metabolic syndrome. Psoriasis patients with insulin resistance or risk factors for metabolic syndrome should be specifically evaluated for fatty liver by abdominal ultrasound and liver biochemistry. Concurrent psoriasis and NAFLD, and the likely synergy between them, affect the general recommendations we can make and the treatment strategies we can adopt. Some drugs carry risk of hepatotoxicity. On the other hand, there is a possibility that achieving good control of inflammation and pathogenic factors associated with NAFLD will improve the prognosis.
- -
The incidence of NAFLD is 1.5- to 3-fold higher in psoriasis patients than in the nonpsoriatic population, meaning we can expect to find NAFLD in roughly half of our patients with psoriasis. The prevalence of steatohepatitis is also higher in psoriasis.
- -
Prognosis in NAFLD is affected by the presence and severity of steatohepatitis and liver fibrosis.
- -
Abdominal ultrasound screening is recommended in patients with insulin resistance or signs of metabolic syndrome and moderate to severe psoriasis, even in the absence of abnormal liver enzymes.
- -
Patients with NAFLD should be referred to a specialist for evaluation and follow-up.
- -
A fatty liver limits therapeutic options and makes it advisable to tailor monitoring in patients taking potentially hepatotoxic drugs.
- -
Anti-TNF biologics offer theoretical benefits for patients with NAFLD as a result of better control of the inflammatory process and peripheral insulin resistance. Ustekinumab and secukinumab do not seem to affect NAFLD adversely.
Risk Factors for Metabolic Syndrome a
| Waist circumference ≥94cm (♂),≥80cm (♀) |
|---|
| Blood pressure≥130/85mmHg or on antihypertensive treatment |
| Fasting glucose≥100mg/dL or in treatment for type 2 diabetes mellitus |
| Triglycerides≥150mg/dL |
| HDL cholesterol<40mg/dL (♂) or<50mg/dL (♀) |
Abbreviation: HDL, high-density lipoprotein.
Source, joint guidelines of the European Association for the Study of the Liver, the European Association for the Study of Diabetes, and the European Association for the Study of Obesity.17
The authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestAbbvie sponsored the meetings of the working group, but no employees of the laboratory participated in the research, interpretation, discussion, or drafting of the text.
J. M. Carrascosa has served as a consultant, given invited talks, and/or participated in clinical trials sponsored by the following laboratories: Abbvie, Janssen, Lilly, Pfizer, Novartis, Pfizer, and Celgene.
Please cite this article as: Carrascosa JM, Bonanad C, Dauden E, Botella R, Olveira-Martín A, en nombre del Grupo de Trabajo en Inflamación Sistémica en Psoriasis. Psoriasis e hígado graso no alcohólico. Actas Dermosifiliogr. 2017;108:506–514.