The prevalence of antiphospholipid antibodies (APLAs) has been extensively studied in patients with systemic lupus erythematosus (SLE) but not in those with cutaneous lupus erythematosus (CLE). We determined the prevalence of APLAs among our patients with CLE, and analyzed their clinical and serologic characteristics.
Materials and methodsThis retrospective study analyzed 182 patients with subacute or chronic CLE who had been in follow-up for 5 years. We selected those positive for 1 or more of the following APLAs in 2 measurements at least 12 weeks apart: lupus anticoagulant (LA), anticardiolipin antibodies (ACAs), and anti-β2-glycoprotein I (anti-β2-GPI) antibodies. In the case of ACAs and anti-β2-GPI antibodies, only patients with titers greater than or equal to 40 U/mL were selected.
ResultsWe obtained a series of 13 patients (4 with subacute disease and 9 with chronic disease). Seven met the diagnostic criteria for SLE and only 1 met the diagnostic criteria for antiphospholipid syndrome (APS). The prevalence of APLAs was 38% among patients with SLE and 3.65% among those without SLE. The most prevalent APLA was LA, present in 10 patients. Antinuclear antibodies (ANAs) were detected in 12 patients and anti-double-stranded DNA antibodies in 11.
ConclusionsThe prevalence of APLAs among our patients with CLE who did not meet the diagnostic criteria for SLE was similar to that reported in the general population. This, along with the strong assocation between the presence of ANAs and the presence of APLAs, would bring into question the value of determining APLAs in patients with CLE who are negative for ANAs. We also note that there was a high prevalence of discoid lesions but a low prevalence of APS among our patients with CLE who were positive for APLAs.
La prevalencia de anticuerpos antifosfolípido (AcAF) en pacientes con lupus eritematoso sistémico (LES) ha sido muy estudiada, pero no en pacientes con lupus eritematoso cutáneo (LEC). Determinamos la prevalencia de AcAF entre nuestros pacientes con LEC, y analizamos sus características clínicas y serológicas.
Material y MétodosEstudio retrospectivo de 182 pacientes con LEC subagudo (LECS) o crónico (LECC) que se hallaban en seguimiento en los últimos cinco años. Seleccionamos a aquellos que presentaban uno o varios de los siguientes AcAF: anticoagulante lúpico (AL), anticuerpos anticardiolipina (ACA) y anticuerpos anti β2-glucoproteína I (anti-β2-GPI), en dos determinaciones, distanciadas al menos en 12 semanas. En el caso de los ACA y los anti-β2-GPI sólo se incluyeron pacientes con titulaciones iguales o superiores a 40 unidades por mililitro.
ResultadosObtuvimos una serie de 13 pacientes: 4 fueron clasificados como LECS y 9 como LECC. Siete cumplían criterios de LES y sólo uno cumplía criterios de SAF. La prevalencia de AcAF fue del 38% entre los que cumplían criterios de LES, y del 3,65% entre los que no los cumplían. El AcAF más prevalente fue el AL, presente en 10 pacientes. Se detectaron Ac ANA en 12 pacientes y anti-dsDNA en 11.
ConclusionesLa prevalencia de AcAF entre nuestros pacientes con LEC que no cumplían criterios de LES fue similar a la referida para la población general. Esto junto a la fuerte asociación de la presencia de ANA y AcAF cuestionaría la rentabilidad de determinar los AcAF en aquellos pacientes con LEC y ANA negativo. Además destaca que entre nuestros pacientes con LEC y AcAF existe una alta prevalencia de lesiones discoides y el desarrollo de SAF es poco frecuente.
Antiphospholipid antibodies (APLAs) are a heterogeneous group of immunoglobulins that target membrane phospholipids or phospholipid-protein complexes involved in the occurrence of thrombotic events and recurrent pregnancy loss in patients with antiphospholipid syndrome (APS).1
There are various APLAs, but only lupus anticoagulant (LA), anticardiolipin antibodies (aCL), and anti-β2-glycoprotein I antibodies (anti-β2GPI) are routinely identified in laboratory tests and form part of the diagnostic criteria for APS.2
APLAs can be found in between 1% and 5% of healthy individuals, but prevalence increases with age and the presence of concomitant chronic disease.3
APLAs appear mainly in association with connective tissue diseases such as systemic lupus erythematosus (SLE)–in fact, they form part of the diagnostic criteria for SLE4–but they are also found in other situations.5 Various infectious agents, tumors, and drugs can cause APLAs to appear transiently, usually without the appearance of anti-β2GPI and only rarely in association with thrombotic tendency.6,7Various studies have investigated the presence of APLAs in patients with SLE and reported prevalence figures ranging from 24%8 to 60%.9 However, the presence of APLAs in patients with the various clinical variants of cutaneous lupus erythematosus (CLE) has been investigated by few studies, and the results have been conflicting.10–13
The objectives of this study were to determine the prevalence of APLAs in a group of patients with subacute CLE or chronic CLE and to analyze their clinical and serologic characteristics.
Materials and MethodsIn this longitudinal retrospective study, we identified in our database 182 patients with SLE who had been in follow-up in our department from January 2006 to December 2010.
Patients who met the following 2 criteria were selected for the study:
- 1.
Presence of 1 or more of the following APLAs: LA (identified by means of hemostasis testing with an ACL TOP 500 system and a silica clotting time test), immunoglobulin (Ig) G or IgM aCL, and IgG or IgM anti-β2GPI. The patients had been tested for each of these antibodies on 2 occasions at least 12 weeks apart, and those with a positive result in both tests were included in the study. In the case of aCL and anti-β2GPI, only patients with titers of 40 U/mL or higher were selected, in accordance with the current diagnostic criteria for APS.2
- 2.
Presence of skin lesions specific to subacute or chronic CLE. Patients who during the course of their illness had only had acute skin lesions were excluded from the study.
Skin lesions were defined according to clinical and histologic criteria included in the classification proposed by Gilliam and Sontheimer14 and modified by Walling and Sontheimer15: a) subacute CLE (annular or papulosquamous/psoriasiform subacute CLE); and b) chronic CLE (discoid LE [localized or disseminated], LE tumidus, hypertrophic LE, LE profundus [panniculitis], lupus pernio, or mucosal discoid LE).
Patients with more than 1 type of skin lesion specific to lupus over the course of their illness were classified according to the first lesion they developed.16
Based on the above criteria, we obtained a series of 13 patients. We studied their clinical characteristics (age, sex, type and location of lupus-specific lesions, other associated cutaneous manifestations, systemic manifestations, and clinical manifestations associated with APS) and serologic characteristics (presence of antinuclear antibodies [ANAs], anti-double-stranded-DNA antibodies [anti-dsDNA], anti-Ro antibody [anti-Ro], anti-La antibody [anti-La], anti-ribonucleoprotein antibody [anti-RNP], and anti-Sm antibody [anti-Sm]; and type of APLA present).
We also determined which patients met the diagnostic criteria for SLE proposed by the American Rheumatism Association (ARA)17,18 and the 2006 Sydney criteria for definitive APS.2
ResultsWe detected the presence of APLAs in 13 of our 182 patients. Among the 18 patients who met the criteria for SLE the prevalence of APLAs was 38.8% (7 patients), whereas among the remaining 164 patients, who did not meet the criteria for SLE, the prevalence of APLAs was only 3.65% (6 patients).
To study the clinical and serologic characteristics of the 13 patients with APLAs, we divided this group into 2 groups: those who met the criteria for SLE and those who did not.
Patients Who Met the Criteria for SLE (Table 1)Epidemiologic DataFive women and 2 men, with a mean age of 43.8 years and a mean disease duration of 15.85 years, met the criteria for SLE.
Clinical and Serologic Characteristics of the Patients Who Met the Criteria for Systemic Lupus Erythematosus.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Sex | Female | Male | Female | Male | Female | Female | Female |
| Age, y | 36 | 36 | 59 | 36 | 42 | 48 | 50 |
| Disease duration, y | 19 | 20 | 19 | 18 | 11 | 9 | 5 |
| Lupus-specific cutaneous manifestations | Subacute annular lesions | Subacute psoriasiform lesions | Subacute psoriasiform | ||||
| Localized chronic discoid lesions | Localized chronic discoid lesions | Disseminated chronic discoid lesions | Disseminated chronic discoid lesions | Disseminated chronic discoid lesions | Disseminated chronic discoid | Chronic | |
| Scarring alopecia | Scarring alopecia | Scarring alopecia | Scarring alopecia | Localized discoid | |||
| Other cutaneous manifestations | Malar erythemaPhotosensitivityOral ulcersDigital ulcersRaynaud phenomenonDiffuse alopecia | Diffuse alopecia | Diffuse alopecia | Malar erythemaPhotosensitivityOral ulcersDigital ulcersRaynaud phenomenon | Diffuse alopecia | ||
| Systemic manifestations | HematologicArticular | Articular | Hematologic | HematologicArticular | HematologicRenalArticularPulmonary | Articular | Renal manifestations |
| Clinical manifestations of APS | – | – | – | – | – | – | – |
| Serologic abnormalities | ANA+ | ANA+ | ANA+ | ANA+ | ANA+ | ANA+ | ANA+ |
| Anti-dsDNA+ | Anti-dsDNA+ | Anti-dsDNA+ | Anti-dsDNA+ | Anti-dsDNA+ | Anti-dsDNA+ | Anti-dsDNA+ | |
| – | – | Anti-Ro+ | Anti-Ro+ | Anti-Ro+ | – | Anti-Ro+ | |
| LA+ | LA+ | LA+ | LA+ | ACA IgM+ | LA+ | LA+ | |
| IgM aCL+ | IgG ACAs+ |
Abbreviations: ANA, antinuclear antibodies; anti-dsDNA, anti-double stranded DNA antibodies; anti-Ro, anti-Ro antibodies; APS, antiphospholipid syndrome; IgG aCL, immunoglobulin G anticardiolipin antibodies; IgM aCL, immunoglobulin M anticardiolipin antibodies; LA, lupus anticoagulant; SLE, systemic lupus erythematosus.
Three patients initially presented subacute lesions (annular in 1 patient and psoriasiform in 2) and later developed chronic discoid lesions (localized in 1 patient and disseminated in 2). Discoid lesions appeared at the onset of the disease in 4 patients (localized in 2 and disseminated in 2). Some patients also had other cutaneous manifestations not specific to LE (Table 1).
Systemic ManifestationsFour patients had hematologic involvement (thrombocytopenia in 3 and hemolytic anemia in 1), 2 had renal involvement (proteinuria in 1 and grade IIB glomerulopathy in 1), 5 had arthritis, and 1 had pleuritis.
No clinical signs or symptoms associated with APS were observed in any of the 7 patients.
Serologic FindingsAll 7 patients were positive for ANAs and anti-dsDNA. The most prevalent APLA in this group was LA, which was present in 6 patients, followed by IgM aCL, present in 2 patients. None of the patients were positive for anti-β2GPI. Two patients were positive for more than 1 APLA.
Patients Who Did Not Meet the Criteria for SLE (Table 2)Epidemiologic DataFour women and 2 men, with a mean age of 45.5 years and a mean disease duration of 5.33 years, did not meet the criteria for SLE.
Clinical and Serologic Characteristics of the Patients Who Did Not Meet the Criteria for Systemic Lupus Erythematosus.
| Patient | 8 | 9 | 10 | 11 | 12 | 13 |
| Sex | Female | Female | Male | Male | Female | Female |
| Age, y | 53 | 33 | 38 | 37 | 61 | 51 |
| Disease duration, y | 3 | 3 | 2 | 6 | 7 | 11 |
| Lupus-specific cutaneous manifestations | Subacute annular lesions | |||||
| Chroniclesions Localized discoid lesions Scarring alopecia | Chroniclesions Disseminated discoid lesions | Chroniclesions Localized discoid lesions Scarring alopecia | Chroniclesions Tumid lesions | Chroniclesions Disseminated discoid lesions | ||
| Other cutaneous manifestations | – | – | – | – | – | Alopecia areata |
| Systemic manifestations | – | – | – | – | – | – |
| Clinical manifestations of APS | Miscarriage in the fourth month of gestation | – | – | – | – | – |
| Serologic abnormalities | ANA+ | ANA+ | ANA+ | ANA+ | ANA+ | – |
| Anti-dsDNA+ | Anti-dsDNA+ | Anti-dsDNA+ | Anti-dsDNA+ | – | – | |
| Anti-Ro+ | – | – | – | Anti-Ro+ | – | |
| – | – | – | – | Anti-La+ | – | |
| LA+ | LA+ | LA+ | LA+ | |||
| IgG aCL+ | IgG aCL+ | IgG aCL+ | IgM aCL+ | |||
| IgG anti-β2GPI+ |
Abbreviations: ANA, antinuclear antibodies; anti-dsDNA, anti-double-stranded-DNA antibodies; anti-La, anti-La antibodies; anti-Ro, anti-Ro antibodies; APS, antiphospholipid syndrome; IgG aCL, immunoglobulin G anticardiolipin antibodies; IgM aCL, immunoglobulin M anticardiolipin antibodies; IgG anti-β2GPI, immunoglobulin G anti-β2-glycoprotein I antibodies; LA, lupus anticoagulant; SLE, systemic lupus erythematosus.
One patient had subacute annular lesions and 5 patients had chronic lesions (discoid in 4 cases and tumid in 1). One patient also had a cutaneous manifestation not specific to LE (Table 2).
Systemic ManifestationsSystemic manifestations were virtually nonexistent in this group. Some of the patients had joint pain but none had arthritis.
One patient had had a miscarriage–a clinical sign of APS–in the fourth month of gestation; she was the only patient who met the criteria for APS. Clinical findings included a plaque of scarring alopecia on the scalp that was smooth and shiny in the center and had scaly, erythematous edges. In addition, in the left malar area there was an erythematous-violaceous, edematous plaque, which healed leaving slightly atrophic skin with residual hyperpigmentation.
Serologic FindingsFive patients were positive for ANAs and 4 for anti-dsDNA. As in the other group, the most prevalent APLA was LA, which was present in 4 patients, followed by IgG aCL, present in 2 patients. Only the patient who met the criteria for APS was positive for IgG anti-β2GPI.
DiscussionVarious studies have analyzed the presence of APLAs in series of patients with SLE and the prevalence figures they report vary widely, from 24%8 to 60%.9 One of the more recent studies, published by Petri4 in 2010, found that 47% of patients were positive for aCL, 32.5% for anti-β2GPI, and 26% for LA. These studies analyze multiple clinical variables but give little attention to skin lesions.
Moreover, the few studies that have analyzed the presence of APLAs in patients with CLE report highly disparate results, possibly because different methodologies were used in each study. In most of the studies, the patients were tested for antibodies just once rather than on 2 occasions at least 12 weeks apart, as is currently recommended.2 In addition, the antibody titer values considered to be positive varied from one study to the next and in many cases were lower than the cutoff levels specified in the current diagnostic criteria for APS.2 We therefore believe that the prevalence of APLAs among patients with CLE is overestimated.
The earliest relevant studies are from 1992. Tebbe and Orfanos10 studied 67 patients, who were divided into 3 groups: a) those who met the criteria for SLE, b) those who did not meet the criteria for SLE and had chronic skin lesions, and c) those who did not meet the criteria for SLE and had subacute skin lesions. The patients were tested for aCL only once, and antibody titer values much lower than the currently recommended cutoff levels were considered positive. A high prevalence–nearly 40%–was found, without significant differences between the groups. In a study published the same year, Kind et al.11 found aCL in 3 (5.8%) of 51 patients with CLE, considering titer values of 10 U/mL or greater to be positive.
Also in 1992, Fonseca et al.12 studied a group of 44 patients with subacute CLE. They tested the patients for aCL on 2 occasions at least 6 months apart and reported the results in accordance with the recommendations of the Second International Anticardiolipin Standardization Workshop. aCL was detected in 7 patients (16%). A positive aCL result was found in 3 (14.2%) of the 21 patients who met the criteria for SLE and in 4 (17.3%) of the 23 patients who did not.
In 1995, Ruffatti et al.13 studied 28 patients with chronic discoid CLE who did not meet the criteria for SLE. In a single test, 67.8% of the patients were positive for IgG aCL and 50% were positive for IgM aCL.
In a study published in 2010, Meuth et al.19 tested 34 patients with CLE for aCL; 5.9% were positive for IgG aCL and 8.8% were positive for IgM aCL. The method used was not described.
As we have seen, figures on the prevalence of APLAs among patients with CLE vary widely in the literature, ranging from 5.8%11 to 68%.13 We believe that in order to accurately determine the prevalence of APLAs among patients with CLE, standard criteria for positive results must be applied across studies. In our study, the prevalence of APLAs among patients with CLE who met the criteria for SLE was 38.8%, a figure similar to those reported in the literature for patients with SLE.4,8,9 Among those who did not meet the criteria for SLE, however, the prevalence of APLAs was 3.65%, a figure similar to that reported in the general population.3 These data call into question the need to include APLA testing in the initial battery of tests that we perform on all patients diagnosed with CLE.
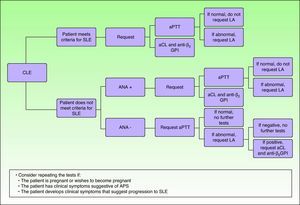
Of the 13 APLA-positive patients in our study, a majority were also positive for ANAs (12 patients) and anti-dsDNA (11 patients). Mayou et al.20 found that aCL was also associated with the presence of ANAs. Because of this association, we believe that APLA tests should be requested for all patients with CLE who receive a positive result for ANAs, which, moreover, is currently among the diagnostic criteria for SLE.18 APLA tests are probably not cost-effective in patients who are negative for ANAs. In Figure 1, we propose a diagnostic algorithm. We believe that patients with CLE who do not meet the criteria for SLE and are ANA-negative should be tested for activated partial thromboplastin time (aPTT). Patients with a prolonged aPTT should be tested for LA, and those who are LA-positive should then be tested for aCL and anti-β2GPI.
Diagnostic algorithm. Abbreviations: CLE indicates cutaneous lupus erythematosus; SLE, systemic lupus erythematosus; ANA, antinuclear antibodies; aPTT, activated partial thromboplastin time; LA, lupus anticoagulant; ACA, anticardiolipin antibodies; anti-β2GPI, anti-β2-glycoprotein I; APS, antiphospholipid syndrome.
A noteworthy clinical finding in our group of patients with APLAs was a high prevalence of discoid lesions. Although the initial diagnosis of subacute or chronic CLE was based on the first lesion to appear, 11 of the 13 patients developed discoid lesions at some point during their illness. Only 2 patients–1 with annular subacute CLE and another with LE tumidus–did not develop such lesions. We have found no similar reports in the literature, but given the small size of our sample we cannot be certain that this finding is significant.
It has been shown that 30% of patients with SLE and APLAs may develop APS within 7 years and that 50% to 70% may develop the syndrome within 20 years.21 In our series, only 1 patient who did not meet the criteria for SLE met the criteria for APS. This patient was a woman with chronic localized discoid CLE lesions who was positive for 3 APLAs (LA, IgG aCL, and IgG anti-β2GPI) and had had a miscarriage in the fourth month of gestation. In previous case series, few patients with CLE and APLAs have developed APS. In a study by Ruffatti et al.,13 only 1 patient with discoid CLE met the criteria for APS. The patient was a man with pulmonary thromboembolism who was positive for IgG aCL, IgM aCL, and LA. Mayou et al.,20 on analyzing the prevalence of ANAs and aCL in a group of 52 patients with discoid CLE, found that aCL was present at low titers and was not associated with increased thrombotic tendency. We cannot rule out the possibility that the low prevalence of APS in our series might be due to the follow-up time, which was 15.5 years in the patients who met the criteria for SLE but only 5.33 years in those who did not. A longer follow-up time would be needed in order to determine the true prevalence of APS in our patients.
ConclusionsThe prevalence of APLAs among our patients with subacute or chronic CLE who met the criteria for SLE was 38.8%, a figure similar to that found in patients with SLE. As expected, the prevalence of APLAs in patients with CLE but without systemic involvement or associated immunologic alterations was no greater than that reported in the general population. However, when the lesions, whether chronic or subacute, are accompanied by a positive test result for ANAs, even in the absence of systemic disease, the likelihood of detecting APLAs is greater and routine testing would be justified.
It is also noteworthy that discoid lesions were very common in patients with CLE and APLAs, and that few of these patients developed APS.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: García-Martín P, et al. Prevalencia de anticuerpos antifosfolípido en pacientes con lupus erite-matoso cutáneo subagudo y crónico. Actas Dermosifiliogr. 2013;104:232–8