Photodynamic therapy (PDT) is a therapeutic modality with significant antimicrobial activity. We present 2 cases of chronic lower limb ulcers in which fungal and bacterial superinfection complicated management. PDT with methylene blue as the photosensitizer led to clinical and microbiological cure with no significant adverse effects. PDT with methylene blue is a valid option for the management of superinfected chronic ulcers, reducing the use of antibiotics and the induction of resistance.
La terapia fotodinámica tiene una importante acción antimicrobiana. Se presentan 2 casos clínicos de úlceras crónicas en las extremidades inferiores sobreinfectadas por hongos y bacterias de difícil manejo, en los que el tratamiento fotodinámico con azul de metileno como fotosensibilizante resultó en la curación clínica y microbiológica. No se presentaron efectos adversos reseñables. La posibilidad de utilizar la terapia fotodinámica con azul de metileno en el manejo de las úlceras crónicas sobreinfectadas constituye una interesante alternativa para reducir el uso de antibióticos y disminuir la aparición de resistencias.
Photodynamic therapy (PDT) is a therapeutic modality based on photooxidation of biological materials induced by a photosensitizer, which is selectively located in some cells which are destroyed on irradiation with a certain wavelength of light.1
PDT has an approved indication for the treatment of keratinocyte skin cancer, although it can have other applications.1,2 In addition to acting on tumor cells, antimicrobial effects of PDT have been known for more than 100 years.3 The advantages of antimicrobial PDT (aPDT) include the possibility to use in mixed infections (not just for different bacteria, whatever their resistance profile, but also for combinations of bacteria, fungi, virus, and even parasites).1,2 It can therefore serve as a complement to conventional antimicrobial agents.2
Given aPDT has a direct local action, it is used above all in accessible areas such as skin lesions and it constitutes a safe therapy with few side effects.1
Colonized and/or infected skin ulcers are an important concern, both from the clinical standpoint (recalcitrant, slow healing, painful, antiesthetic, source of infection) and the epidemiological one (reservoir of multiresistant bacteria and hard-to-treat fungi), not to mention the extra costs associated with expensive treatments.4
PDT has other actions in addition to the improvement that may result from a decrease, or disappearance, of the microbial load.5 It can stimulate healing and skin epithelialization through immunoregulatory and regenerative processes, such as increased hydroxyproline content, improved angiogenesis, and modulation of collagen (attenuating its degradation and mediating remodelling).5–7
Practical experience in the use of aPDT in the management of ulcers is limited, although 2 clinical trials have been published with promising results.8,9 Our group has experience with the resolution of a complex case of recalcitrant ulcerative cutaneous sporotrichosis, treated with PDT with intralesional methylene blue (MB) in combination with a systemic antifungal agent.10
We present 2 cases of superinfected nonhealing leg ulcers that resolved with the help of PDT in its 2 modalities: conventional application (with the Aktilite lamp) and with exposure to daylight.
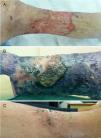
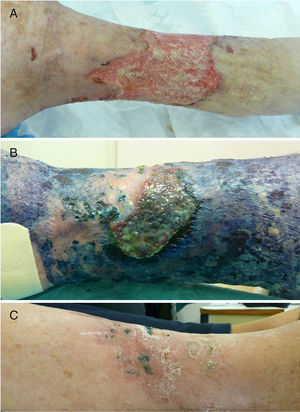
Case HistoriesCase 1A 76-year-old woman was admitted to the internal medicine department with chronic malodorous grade iv varicose vein ulcer on the right leg. The course of the lesion was nonhealing and extending with suppuration and necrosis (Fig. 1A). Fusarium species and Pseudomonas aeruginosa grew rapidly in the cultures. Surgical debridement was performed and the patient treated empirically with linezolid and piperacillin-tazobactam iv. After the findings of the culture were available, treatment was switched to ciprofloxacin and voriconazole was added. The lesion was very refractory to treatment, with multiple complications (urinary sepsis and stroke) and new concurrent treatments were initiated (anticoagulants, ceftazidime, and amikacin). In the face of this clinical course, and after obtaining informed consent from the patient, PDT was applied with 1% MB solution (30min of incubation under occlusion) and, after rinsing away any excess MB, irradiation with the Aktilite lamp (Galderma SL, France; fluence of 37J/cm2). Three sessions were administered in the first week and 2 in the second and third weeks (Fig. 1B). The improvement was notable and the patient discharged. She continued with 2 weekly sessions of PDT with daylight at home (MB application and exposure to daylight for 30min daily, with fortnightly follow-up). Late follow-up revealed methicillin-resistant Staphylococcus aureus (MRSA) in the culture. This was considered a colony of little pathogenic importance, although topical mupirocin was applied and home PDT continued. The bacterium was not detected in the next microbiological control. After 6 months of treatment, complete healing of the lesion was achieved (Fig. 1C).
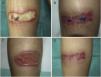
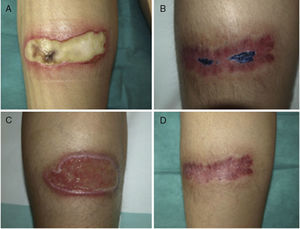
Case 2A 38-year-old woman with a combined kidney and pancreas transplant, in treatment with tacrolimus and prednisone, suffered symmetric burns to the calf area during a surgical procedure for eventration. On visual inspection, 2 symmetric ulcers were observed of 9×4cm, which initially were covered by a blackish sore (Fig. 2 A and C). Corynebacterium striatum and Fusarium oxysporum were isolated repeatedly from the lesions. The patient was treated with oral ciprofloxacin for a week and PDT was initiated with 1% MB solution according to the protocol described above. One session weekly was administered, and culture at 8 weeks was negative for bacteria and fungi. After 2 months, healing of the right side was observed, whereas MSRA was isolated from the left side. PDT with MB was restarted with mupirocin administered between sessions. Culture was negative for MRSA after 3 weeks. After 3 months, the 2 lesions had healed (Figs. 2 B and D).
During the course of infection, cultures became negative in both cases and there was a gradual decrease in microbial load (bacteria and fungi). The perception of the patients was very positive, with the therapy being subjectively assessed as very effective and painless. The only side effect reported was the intense blue coloration of the treated area and, occasionally, of urine.
Discussion and ConclusionPDT with MB was effective in the management of 2 non-healing ulcers superinfected with Fusarium species, Pseudomonas and other difficult-to-treat bacteria. The 2 patients achieved healing and epithelization of the ulcers without the need for costly therapies that are not free of interactions and adverse effects and that may require parenteral administration.
The presence of Fusarium species in both cases and of P aeruginosa in the first patient represents a paradigm of microorganisms that are difficult to treat with anti-microbial agents. P aeruginosa in particular is currently a therapeutic challenge because of its increasing multiresistance. In addition, MRSA was isolated in both cases during the clinical course of the lesion but systemic treatment was not required and the infection could be treated with topical mupirocin and PDT itself. PDT can be considered a safe, simple, and inexpensive adjuvant therapy that, in addition, does not lead to resistance, has a broad spectrum of action, and may have an effect on the biofilm.11 The phenomenon of biofilm or biolayer formation appears to play a role in delayed wound healing. The process consists of the formation of aggregated bacterial communities covered by an extracellular matrix of polysaccharides that protects against antibiotics and the patient's immune system.12 PDT complements antimicrobial treatments, with which it may have synergistic effects,11 avoids expensive and aggressive therapies for the microbiota of the patients, and is free of interactions, and so can be particularly useful in immunosuppressed patients, patients with diabetes, and multimedicated elderly patients, as described here.13 Moreover, when applied to skin ulcers, PDT appears to reduce the microbial load and substantially promote healing.11
Biopsy was not performed in either of the patients as, given the natural course of the lesions, there were no grounds for clinical suspicion of another cause (for example, pyoderma gangrenosum).
No approved photosensitizers are available on the market for aPDT. Different in vitro and in vivo studies show that MB has an excellent profile with activity against gram-positive and gram-negative bacteria and fungi.2 In the case of Pseudomonas, there are several reports of successful application of PDT for skin lesions, both in in vitro and in animal models.14–16 The cases presented here are the first experience in the use of PDT with MB in Fusarium species.
MB is indicated for human use with intravenous administration, and so it is safe. It is also cheap and already available in the hospital. Nevertheless, precautions are necessary, even when used topically in aPDT, for example, in the case of pregnancy and patients treated with serotonergic psychiatric drugs (http://www.fda.gov/Drugs/DrugSafety/ucm267886.htm).
aPDT acts only when the photosensitizer is photoactivated, and as soon as there is no light source, it is no longer active. For this reason, we recommend that patients continue to activate MB that persists in the ulcer for several days after application through exposure to sunlight. This enables a daily dose of PDT to be applied.
In conclusion, the cases presented are an example of how aPDT can be a therapeutic alternative with a promising future in the management of chronic skin ulcers. Use of this technique can help overcome multiple resistance and antimicrobial agents can be reserved for patients with infections that can be effectively treated with such agents and that are at sites where PDT is not effective.
Ethical ResponsibilitiesProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data.The authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consent.The authors declare that patient data do not appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
FundingThe authors received the support of the Ministry of Economy, Industry and Competitiveness in their investigation into photodynamic therapy through the Spanish National Plan for Scientific and Technical Research and Innovation CTQ2013-48767-C3-2-R. They also received support from the Government of Aragon through participation in the B-85 Research Group.
The authors would like to express their thanks to Dr. Antonio Rezusta for his collaboration and guidance.
Please cite this article as: Aspiroz C, Sevil M, Toyas C, Gilaberte Y. Terapia fotodinámica con azul de metileno en úlceras cutáneas infectadas con Pseudomonas aeruginosa y Fusarium spp. Actas Dermosifiliogr. 2017;108:e45–e48.