In the developed world, pellagra is a rare condition that is restricted to a small number of at-risk groups. It mainly affects alcoholic patients and those with dietary deficiencies, with intestinal malabsorption, or in treatment with certain drugs. The aim of this study was to analyze the clinical, histopathological, and epidemiological characteristics of patients diagnosed with pellagra in our hospital and to compare the results with the findings traditionally described for this disease.
Patients and methodsWe undertook a retrospective study of patients with clinical or pathological evidence of pellagra who were seen in our hospital between 1998 and 2009.
ResultsSeven patients met the inclusion criteria. All were men and the most common predisposing factors were alcoholism and dietary deficiency. All exhibited photosensitivity mainly affecting the forearms and the upper surface of the feet, where the lesions were more severe. The most consistent histopathological findings were the presence of dilated blood vessels with extravasation and little or no inflammatory infiltrate. Various changes were observed in the epidermis, including those suggestive of mild pellagra, such as epidermal pallor and some degree of ballooning of the keratinocytes. Other abnormalities such as epidermal necrosis and hyperkeratosis were also observed. In most patients, pellagra was not initially suspected. Additional noncutaneous findings were observed in almost all cases.
ConclusionsPellagra should be ruled out in patients with lesions on sun-exposed areas. Predisposing factors for pellagra should be assessed along with the social situation of patients and the presence of digestive or neurological abnormalities.
En el mundo desarrollado la pelagra es una entidad rara confinada a unos pocos grupos de riesgo. Afecta especialmente a personas alcohólicas, con transgresiones dietéticas, malabsorción intestinal o en tratamiento con determinados medicamentos. El objetivo del presente trabajo es realizar un estudio de las características clínicas, histopatológicas y epidemiológicas de los pacientes diagnosticados de pelagra en nuestro centro, y compararlo con los hallazgos “clásicos” de esta entidad.
Pacientes y métodosSe realiza un estudio retrospectivo de los pacientes con hallazgos clínicos y/o patológicos de pelagra en nuestro centro en el periodo comprendido entre 1998 y 2009.
ResultadosSiete pacientes cumplían los criterios de inclusión. Todos eran varones y los factores predisponentes más importantes fueron el alcoholismo y la transgresión dietética. Todos mostraban un cuadro de fotosensibilidad, donde el dorso de los antebrazos fue el área más afectada y el dorso del pie la zona donde las lesiones eran más graves. Los hallazgos histopatológicos más constantes fueron la presencia de vasos dilatados asociados a una extravasación hemática, con escaso o nulo infiltrado inflamatorio. Los cambios epidérmicos fueron variados e incluyeron cambios sugestivos de pelagra en grado leve, como una palidez de la epidermis y cierto grado de balonización de los queratinocitos, pero también otras alteraciones como ampollas con necrosis epidérmica e hiperqueratosis. En la mayoría de los pacientes la sospecha clínica inicial no fue de pelagra. Casi todos asociaban una discreta clínica extracutánea.
ConclusionesAnte pacientes con lesiones en áreas fotoexpuestas se debe descartar pelagra. Para ello se deben investigar los factores predisponentes de pelagra, la situación social del paciente y la presencia de alteraciones digestivas y/o neurológicas.
Although pellagra used to be a disease of epidemic proportions in the developed world, it is now a rare condition confined to certain at-risk groups. Little progress has been made in our knowledge of pellagra since it was first described by Gaspar Casal in 1735 and since Goldberger discovered that nicotinamide was a preventive factor in 1926.1,2 Proof of this is that there have been no changes in treatment or diagnostic criteria in the last 90 years. The main difference between now and 90 years ago is probably our limited current experience due to the rarity of the disease. The aim of this study was to report our experience with pellagra in a series of patients at our hospital.
Patients and MethodsWe reviewed the clinical and histologic records of Hospital Doctor José Molina Orosa in Lanzarote, in the Canary Islands, Spain in search of diagnoses of pellagra and pellagra-like syndrome between 1998 and 2009. The inclusion criteria were (a) clinical findings consistent with pellagra (more or less symmetrical skin lesions in sun-exposed areas) or histologic findings consisting of pallor in the upper layers of the epidermis with ballooning of keratinocytes; (b) resolution of symptoms following administration of nicotinamide; and (c) exclusion of other diseases. To be included in the study, patients had to fulfill all 3 criteria.
We reviewed the medical records and noted data on age, sex, predisposing factors for pellagra, substance abuse (in particular excessive alcohol consumption), social situation, clinical manifestations, abnormal laboratory results, initial suspected diagnosis, treatment, and long-term outcome. We also examined the histology reports of patients who underwent biopsy.
Incidence and prevalence data were based on annual hospital records and demographic data were compiled from the council data center of Lanzarote.3
ResultsSeven patients were included in the study. Tables 1–3, respectively, show the corresponding epidemiological, clinical, and histologic data.
Epidemiological Findings.
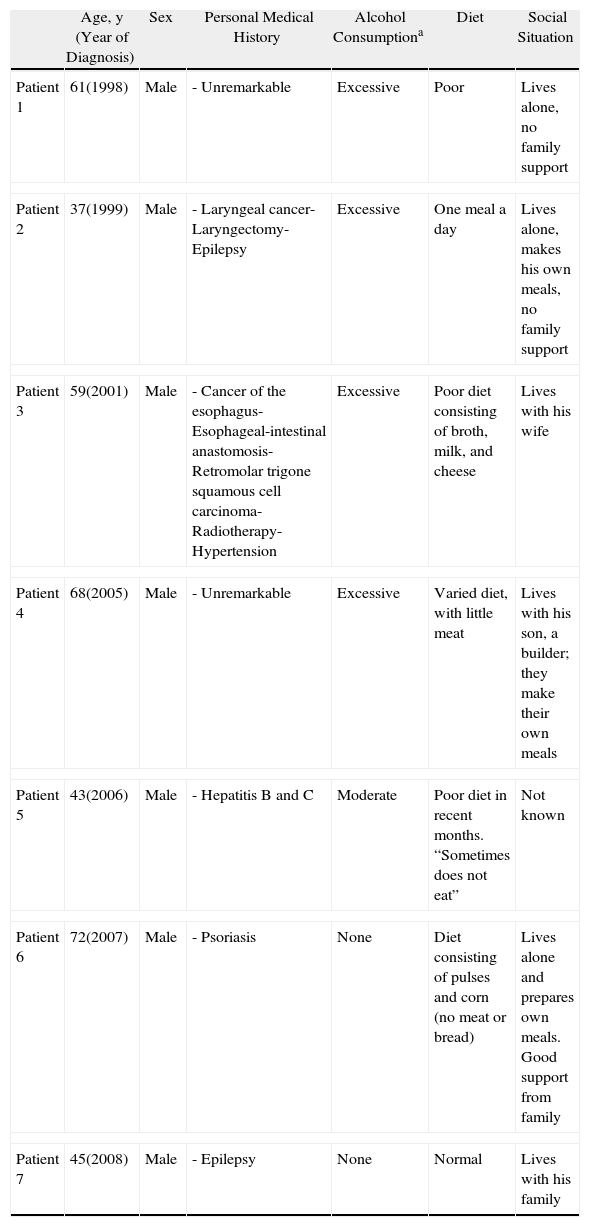
| Age, y (Year of Diagnosis) | Sex | Personal Medical History | Alcohol Consumptiona | Diet | Social Situation | |
| Patient 1 | 61(1998) | Male | - Unremarkable | Excessive | Poor | Lives alone, no family support |
| Patient 2 | 37(1999) | Male | - Laryngeal cancer- Laryngectomy- Epilepsy | Excessive | One meal a day | Lives alone, makes his own meals, no family support |
| Patient 3 | 59(2001) | Male | - Cancer of the esophagus- Esophageal-intestinal anastomosis- Retromolar trigone squamous cell carcinoma- Radiotherapy- Hypertension | Excessive | Poor diet consisting of broth, milk, and cheese | Lives with his wife |
| Patient 4 | 68(2005) | Male | - Unremarkable | Excessive | Varied diet, with little meat | Lives with his son, a builder; they make their own meals |
| Patient 5 | 43(2006) | Male | - Hepatitis B and C | Moderate | Poor diet in recent months. “Sometimes does not eat” | Not known |
| Patient 6 | 72(2007) | Male | - Psoriasis | None | Diet consisting of pulses and corn (no meat or bread) | Lives alone and prepares own meals. Good support from family |
| Patient 7 | 45(2008) | Male | - Epilepsy | None | Normal | Lives with his family |
| Predisposing Factors for Pellagra | |
| Patient 1 | Excessive alcohol consumption |
| Poor diet | |
| Patient 2 | Excessive alcohol consumption |
| Poor diet | |
| Antiepileptic treatment | |
| Laryngeal cancer | |
| Patient 3 | Poor diet |
| Intestinal malabsorption | |
| Excessive alcohol consumption | |
| Patient 4 | Excessive alcohol consumption |
| Patient 5 | Poor diet |
| Moderate alcohol consumption | |
| Patient 6 | Poor diet |
| Patient 7 | Antiepileptic treatment |
Classification of alcohol consumption according to the National Institute on Alcohol Abuse and Alcoholism.22
Clinical Findings and Disease Course.
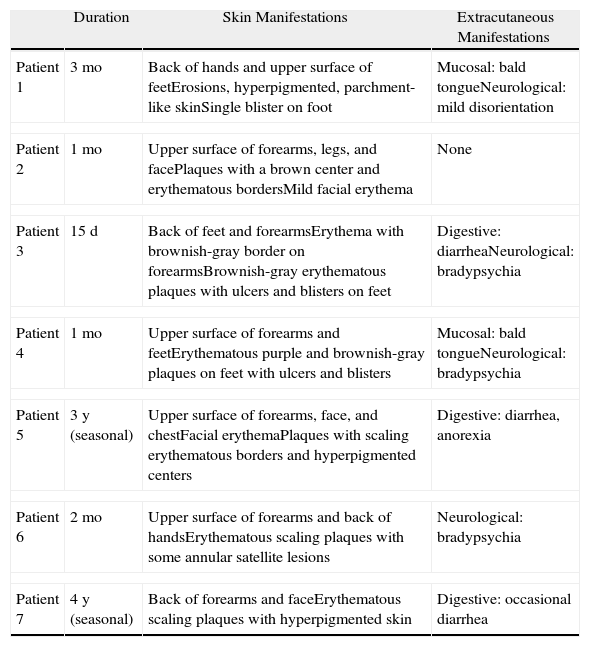
| Duration | Skin Manifestations | Extracutaneous Manifestations | |
| Patient 1 | 3 mo | Back of hands and upper surface of feetErosions, hyperpigmented, parchment-like skinSingle blister on foot | Mucosal: bald tongueNeurological: mild disorientation |
| Patient 2 | 1 mo | Upper surface of forearms, legs, and facePlaques with a brown center and erythematous bordersMild facial erythema | None |
| Patient 3 | 15 d | Back of feet and forearmsErythema with brownish-gray border on forearmsBrownish-gray erythematous plaques with ulcers and blisters on feet | Digestive: diarrheaNeurological: bradypsychia |
| Patient 4 | 1 mo | Upper surface of forearms and feetErythematous purple and brownish-gray plaques on feet with ulcers and blisters | Mucosal: bald tongueNeurological: bradypsychia |
| Patient 5 | 3 y (seasonal) | Upper surface of forearms, face, and chestFacial erythemaPlaques with scaling erythematous borders and hyperpigmented centers | Digestive: diarrhea, anorexia |
| Patient 6 | 2 mo | Upper surface of forearms and back of handsErythematous scaling plaques with some annular satellite lesions | Neurological: bradypsychia |
| Patient 7 | 4 y (seasonal) | Back of forearms and faceErythematous scaling plaques with hyperpigmented skin | Digestive: occasional diarrhea |
| Initial Diagnosis | Laboratory Tests | Treatment | Short-term Outcome | Long-term Outcome | |
| Patient 1 | Pellagra | None | Nicotinamide 100 mg/dSun protection | Resolution | No information |
| Patient 2 | Pellagra | None | Nicotinamide 150 mg/d | Marked improvement after 1 mo | No informationDeath due to laryngeal cancer in 1999 |
| Patient 3 | Pseudoporphyria cutanea tarda | Hb, 9.1; T prot, 4.6; Alb, 2.4; Chol, 132; GGT, 160; Fe 26; Porphyrins, N; 5-OH indoleacetic acid, N | Becozyme C forte: 3 tablets/dHigh-protein diet | Resolution in 1.5 mo | DeathRequired hospitalization due to malnutrition; pellagra did not recur |
| Patient 4 | Pellagra | Chol, 133; Fe, 59; Porphyrins, N; ANA, N; 5-OH indoleacetic acid, N | Becozyme C forte: 3 tablets/d | Resolution | No information |
| Patient 5 | Lupus | Fe, 39; porphyrins, N; 5-OH indoleacetic acid, N | Becozyme C forte: 3 tablets/dSun protection | Resolution in 1.5–2 moWeight gain | No information |
| Patient 6 | Photocontact dermatitis | ANA, N; 5-OH indoleacetic acid, N | Becozyme C forte: 3 tablets/dSun protectionTopical corticosteroids | Resolution | Recurrence after 6 moNo further recurrences |
| Patient 7 | Lupus, polymorphic light eruption | Ferritin, 19; ANA, N; porphyrins, N; 5-OH indoleacetic acid, N | Sun protectionTopical corticosteroids | Resolution | No recurrence |
Abbreviations: Alb, albuminum; ANA, antinuclear antibodies; Chol, cholesterol; Fe, iron, Hb, hemoglobin; N, normal; T prot, total protein.
Histologic Findings.
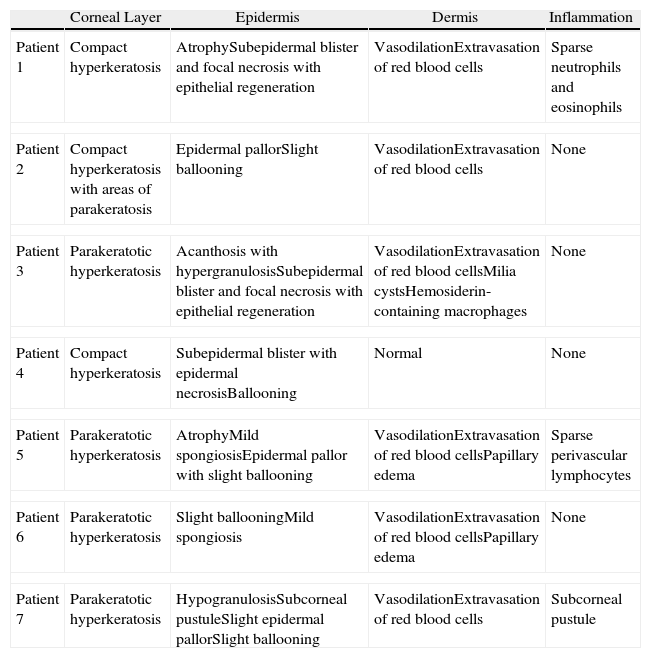
| Corneal Layer | Epidermis | Dermis | Inflammation | |
| Patient 1 | Compact hyperkeratosis | AtrophySubepidermal blister and focal necrosis with epithelial regeneration | VasodilationExtravasation of red blood cells | Sparse neutrophils and eosinophils |
| Patient 2 | Compact hyperkeratosis with areas of parakeratosis | Epidermal pallorSlight ballooning | VasodilationExtravasation of red blood cells | None |
| Patient 3 | Parakeratotic hyperkeratosis | Acanthosis with hypergranulosisSubepidermal blister and focal necrosis with epithelial regeneration | VasodilationExtravasation of red blood cellsMilia cystsHemosiderin-containing macrophages | None |
| Patient 4 | Compact hyperkeratosis | Subepidermal blister with epidermal necrosisBallooning | Normal | None |
| Patient 5 | Parakeratotic hyperkeratosis | AtrophyMild spongiosisEpidermal pallor with slight ballooning | VasodilationExtravasation of red blood cellsPapillary edema | Sparse perivascular lymphocytes |
| Patient 6 | Parakeratotic hyperkeratosis | Slight ballooningMild spongiosis | VasodilationExtravasation of red blood cellsPapillary edema | None |
| Patient 7 | Parakeratotic hyperkeratosis | HypogranulosisSubcorneal pustuleSlight epidermal pallorSlight ballooning | VasodilationExtravasation of red blood cells | Subcorneal pustule |
All the patients were men aged between 37 and 72 years at the time of diagnosis. The mean age was 55 years. The annual incidence was 0.5 cases per 100000 inhabitants. Based on data from the hospital, 1 in every 4663 new patients seen by the dermatology department was diagnosed with pellagra.
The most common predisposing factors were excessive alcohol consumption and poor diet. It is noteworthy that 2 patients were being treated for epilepsy and that this treatment was the only predisposing factor in 1 of these (Table 1).
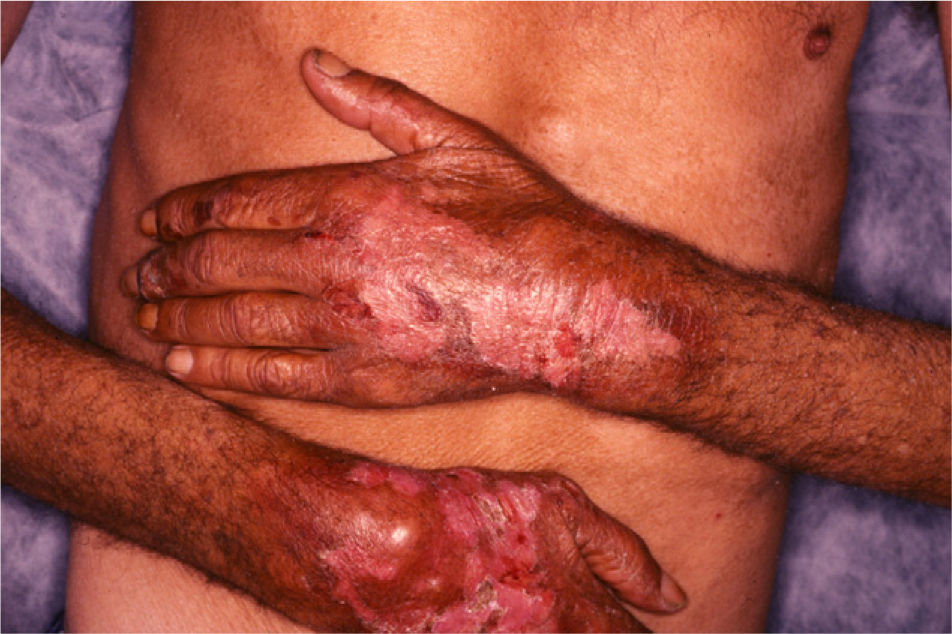
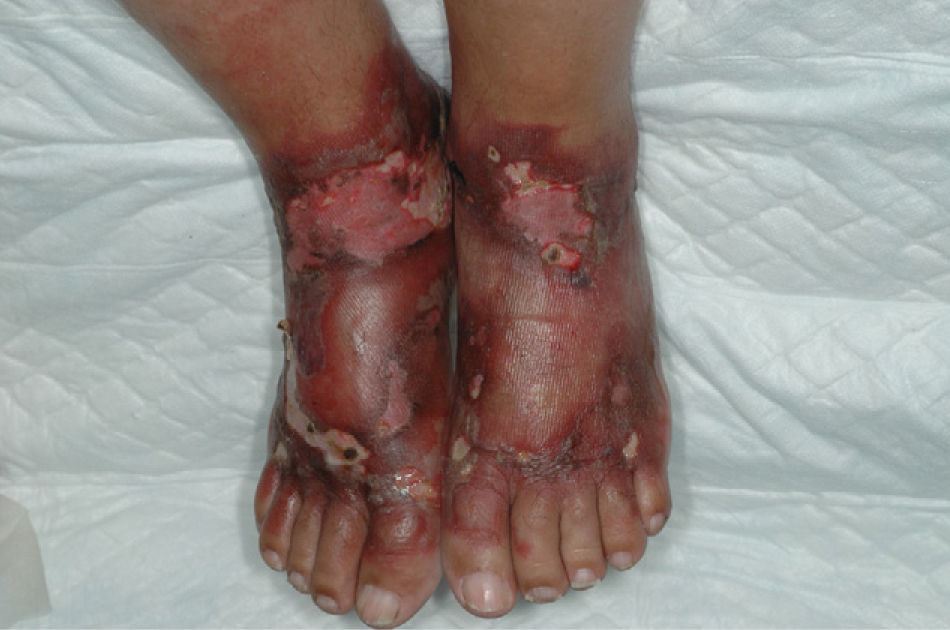
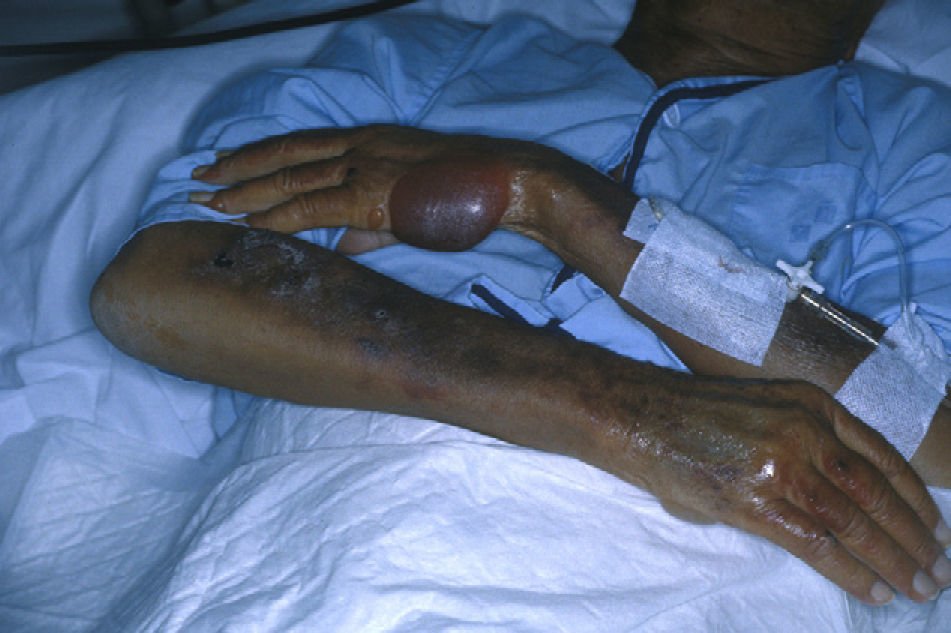
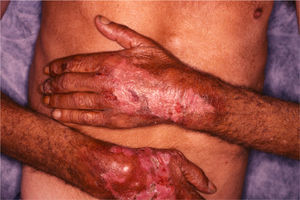
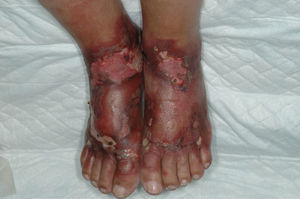
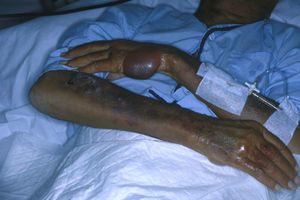
The back of the hands and/or the forearms were affected in all 7 patients (Table 2) and all the lesions were bilateral and symmetrical (Fig. 1). The face and the upper surface of the feet and/or the legs were affected in fewer patients. The lesions on the face were mild, but there were blisters and ulcers on the feet and/or the legs (Figs. 2 and 3).
Pellagra was only suspected initially in 3 patients, even though they all had characteristic clinical manifestations. Other diagnoses considered were lupus, photocontact dermatitis, polymorphic light eruption, and pseudoporphyria cutanea tarda.
Almost all of the patients had extracutaneous symptoms, but these were mild and were only detected during a structured interview. Two patients had an impaired oral mucosa membrane (bald tongue and atrophy of the mucosa). There were also gastrointestinal symptoms (diarrhea) and neurological symptoms (bradypsychia and slight disorientation).
Laboratory tests were only of value for ruling out other diseases.
The outcome was favorable in all patients but resolution was slower in 2 patients as niacinamide treatment was not administered correctly initially.
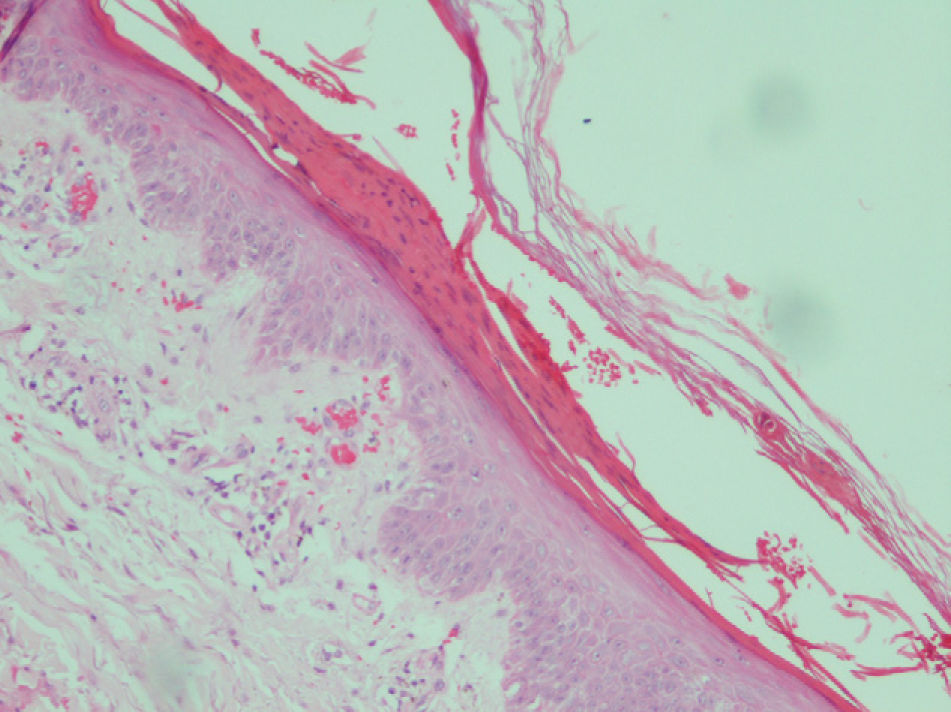
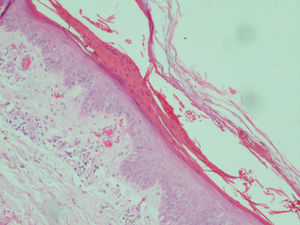
The most common histologic findings were the presence of dilated vessels associated with extravasated red blood cells, little or no inflammatory infiltrate in the dermis, and compact or parakeratotic hyperkeratosis in the epidermis (Table 3). There was some degree of ballooning of keratinocytes and epidermal pallor in 4 cases but it was so slight that it did not raise suspicion of pellagra (Fig. 4). Three patients had subepidermal blisters. A direct immunofluorescence study performed in 1 patient (#3) was negative.
Patient #5. Histologic image showing parakeratotic hyperkeratosis together with a somewhat atrophic epidermis characterized by mild spongiosis, pallor, and ballooning. Dilated vessels with extravasation of red blood cells can be observed in the dermis (hematoxylin–eosin staining, original magnification ×200).
Pellagra is endemic in parts of the developing world and there have also been reports of outbreaks in areas affected by famine or war.4 It is extremely rare in developed countries, where it is limited to a small number of at-risk groups. Its incidence in Spain is unknown as only a few isolated cases and small series have been published.5,6 The only reference is a series described by Rodríguez-Cuartero et al.6 in which 0.23% of patients admitted to a hospital had pellagra. Based on our findings, the annual incidence of pellagra is 0.5 cases per 100000 population.
Pellagra is caused by a deficiency of the coenzymes nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP), which participate in numerous processes including glycolysis, the metabolism of amino acids and proteins, and the generation of high-energy phosphate bonds. This would explain why pellagra mainly affects tissues with a high rate of cell turnover, such as the skin and the digestive tract, and tissues with high energy needs, such as the brain.2,7,8 Humans synthesize NAD and NADP from niacin (or its amide niacinamide), which is absorbed directly through the intestinal wall or synthesized from the essential amino acid tryptophan.
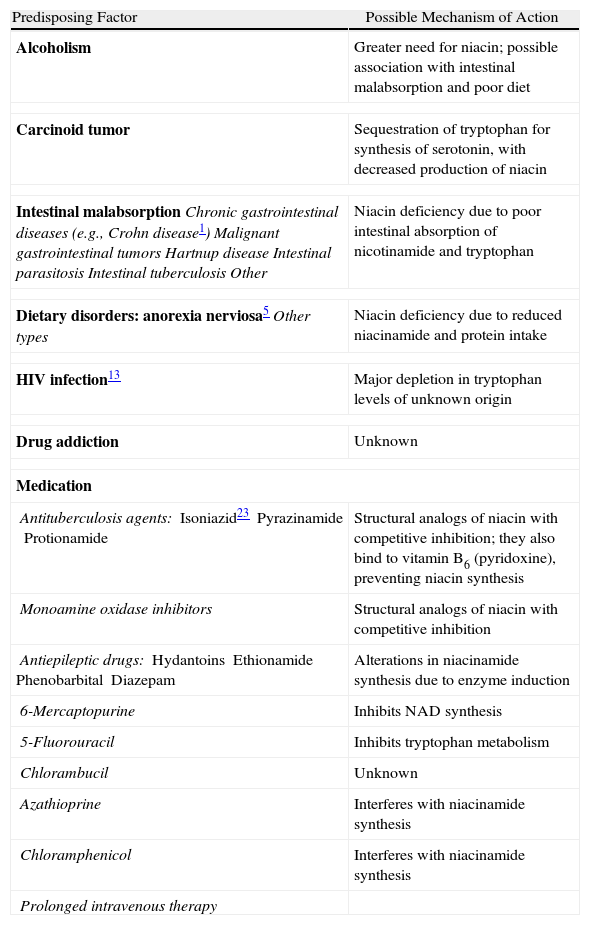
Table 4 shows the predisposing factors for pellagra in the developed world. Alcoholism is the most important factor and patients with excessive alcohol intake probably have dietary deficiencies in addition to a greater need for niacin.9 In our series, 4 of the 5 patients who consumed excessive amounts of alcohol had a poor diet. Depletion of intracellular NAD levels has been observed in patients with human immunodeficiency virus (HIV) infection,10 and these patients also probably have a greater need for niacin.11 Nonetheless, and despite the publication of isolated reports describing an association between niacin depletion and HIV,12,13 HIV-positive patients do not appear to have a higher incidence of pellagra.13,14
Predisposing Factors.
| Predisposing Factor | Possible Mechanism of Action |
| Alcoholism | Greater need for niacin; possible association with intestinal malabsorption and poor diet |
| Carcinoid tumor | Sequestration of tryptophan for synthesis of serotonin, with decreased production of niacin |
| Intestinal malabsorptionChronic gastrointestinal diseases (e.g., Crohn disease1)Malignant gastrointestinal tumorsHartnup diseaseIntestinal parasitosisIntestinal tuberculosisOther | Niacin deficiency due to poor intestinal absorption of nicotinamide and tryptophan |
| Dietary disorders: anorexia nerviosa5Other types | Niacin deficiency due to reduced niacinamide and protein intake |
| HIV infection13 | Major depletion in tryptophan levels of unknown origin |
| Drug addiction | Unknown |
| Medication | |
| Antituberculosis agents:Isoniazid23PyrazinamideProtionamide | Structural analogs of niacin with competitive inhibition; they also bind to vitamin B6 (pyridoxine), preventing niacin synthesis |
| Monoamine oxidase inhibitors | Structural analogs of niacin with competitive inhibition |
| Antiepileptic drugs:HydantoinsEthionamidePhenobarbitalDiazepam | Alterations in niacinamide synthesis due to enzyme induction |
| 6-Mercaptopurine | Inhibits NAD synthesis |
| 5-Fluorouracil | Inhibits tryptophan metabolism |
| Chlorambucil | Unknown |
| Azathioprine | Interferes with niacinamide synthesis |
| Chloramphenicol | Interferes with niacinamide synthesis |
| Prolonged intravenous therapy | |
Other important factors that predispose to pellagra detected in our series were dietary deficiencies and use of antiepileptic drugs. Our findings also suggest that social situation plays an important role in the development of pellagra; 3 of the patients lived alone and a fourth lived with his son, and they were all responsible for preparing their own meals and managing their diet (Table 1).
Although pellagra was traditionally associated with the 3 D's (dermatitis, diarrhea, and dementia)—and possibly with a fourth (death)—this classic clinical pattern is now rare and only some of these manifestations are present.15 In our series, just 1 patient had cutaneous, digestive, and neurological symptoms. Because the digestive and neurological symptoms are varied and nonspecific, pellagra is generally diagnosed on the basis of skin manifestations, which are much more specific.16
The clinical manifestations in our series were quite characteristic of pellagra but this diagnosis was only suspected in 3 of the 7 patients initially. The presence of photosensitivity was recorded in all cases but it raised suspicion of conditions that are more common in our setting. The extracutaneous manifestations, which can help to reach a diagnosis, were not reported spontaneously and were only detected during a structured interview. This is probably because the patients did not associate these symptoms with their skin lesions and did not consider them to be important because of their mild nature.
There are currently no tests or laboratory markers of diagnostic value in pellagra. Indirect signs of malnutrition can help to confirm a diagnosis, but in our case, these were only evident in patient #3, who had marked intestinal malabsorption. Abnormal 5-OH-indoleacetic acid levels in a 24-hour urine test have been proposed as a potential marker for pellagra. Elevated levels may be due to a carcinoid tumor, which is a known risk factor for pellagra, and reduced levels could indicate tryptophan sequestration for the synthesis of niacin, causing depleted serotonin levels. Nonetheless, no cutoff points have been established and in our series at least, determination of 5-OH-indoleacetic acid levels was not of value. Blood levels of NAD and NADP have not been found to correlate well with tissue levels in patients with pellagra as they are not reduced.17 Seal et al.4 measured the levels of the niacinamide metabolites 1-methylnicotinamide and 1-methyl-2-pyridone-5-carboxymide in relation to blood creatinine levels in an Angolan population with and at risk of pellagra. While the metabolites were reduced in patients with pellagra, they were also reduced in 29.4% of the general population, suggesting that determination of these metabolites might be useful for identifying vulnerable populations in developing countries. It remains to be determined, however, whether such testing might be of diagnostic value in the developed world.
The histologic features of pellagra vary throughout the course of the disease and are nonspecific in many cases.18,19 Suggestive signs are prominent pallor and extensive ballooning of keratinocytes in the upper third of the epidermis.20 None of our patients had characteristic histologic features of pellagra. The most common changes observed were hyperkeratosis of the epidermis and dilation of the superficial vascular plexus associated with extravasated red blood cells in the dermis. Subepidermal blistering was observed in 3 cases. It is important to note that blisters in pellagra are generally intraepidermal due to excessive ballooning of keratinocytes,20 but that subepidermal blisters can also form,18,19,21 possibly through a mechanism similar to that seen in sunburn.
Treatment essentially consists of correcting predisposing factors (e.g., suppression of alcohol consumption and prescription of nicotinamide supplementation). Nicotinamide is preferred to nicotinic acid as the latter can cause intestinal disorders, hyperuricemia, and vasomotor disorders.18 The starting dose should be 100mg/6h, followed by 50mg/8h when the patient improves. Zinc, pyridoxine, and magnesium supplements should also be prescribed as these have been seen to act as cofactors in niacin synthesis.2,9
Interestingly, there might be an increased risk of pellagra in the Canary Islands due to the confluence of several factors, including high alcohol consumption, considerable sun exposure, and a diet rich in gofio (toasted cornflour). It should be recalled that the replacement of wheat with corn as a staple food in Spain was the main cause of the pellagra epidemic that swept the country in the 18th century.
Few conclusions can be drawn from our study due to its retrospective design and small sample size, but a number of interesting observations can be made: (1) pellagra is rare but does exist in certain at-risk groups such as individuals with chronic alcoholism or dietary deficiencies, or patients taking certain drugs; (2) it should be included in the differential diagnosis of any condition with photosensitivity; (3) its detection requires a high degree of diagnostic suspicion; and (4) social situation should be considered in the assessment of patients.
Conflict of InterestsThe authors declare that they have no conflicts of interest.
Please cite this article as: Piqué-Duran E, Pérez-Cejudo JA, Cameselle D, Palacios-Llopis S, García-Vázquez O. Pelagra: estudio clínico, histopatológico y epidemiológico de 7 casos. Actas Dermosifiliogr. 2012;103:51–58.