To assess the safety and effectiveness of oral propranolol (OP) in the treatment of infantile hemangiomas.
Material and methodWe conducted a prospective study of infantile hemangiomas (IHs) treated with oral propranolol between October 2008 and March 2011. We included fast-growing IHs in the proliferative phase, IHs affecting vital structures, ulcerated IHs, and IHs that could cause functional or aesthetic problems after the proliferative phase. The patients received oral propranolol 2mg/kg/d and were monitored on an outpatient basis. Response to treatment was assessed by volume reduction, lightening of color, improvement of symptoms, and parent satisfaction. Time of initial and peak response, as well as side effects and sequelae, were recorded.
ResultsWe analyzed 20 IHs, corresponding to 17 girls and 3 boys. The main sites of involvement were around the eyes (20%), the nose (15%), the neck (15%), and the trunk (15%). Ninety percent of the hemangiomas were focal and in the proliferative phase. Treatment was started between the ages of 2 and 19 months and the main reason for starting treatment was rapid growth (50% of cases). Initial response was observed in 70% of cases and only in 2 of them it took over a month. Peak response occurred at 3 months. All the IHs responded to treatment; response was excellent in 55% of cases, good in 35%, and minimal in 10%. The following factors were predictive of response: focal IH, proliferative phase, periorbital location, and ulceration. No serious side effects were observed.
ConclusionOral propranolol was clinically effective in reducing the volume and color of infantile hemangiomas, although the reduction was not complete and telangiectasia and scarring persisted after treatment. Oral propranolol also proved to be safe for use in outpatients.
Valorar la efectividad y seguridad del propranolol oral (PO) para tratar hemangiomas infantiles (HI).
Material y métodosEstudio prospectivo de los HI tratados con PO entre octubre de 2008 y marzo de 2011. Fueron candidatos a tratamiento los HI en fase proliferativa con rápido crecimiento, compromiso de alguna estructura vital o ulceración y para evitar problemas funcionales o estéticos tras la fase proliferativa. Los pacientes se trataron con 2mg/kg/día de PO, y fueron controlados ambulatoriamente. Se valoró la respuesta terapéutica mediante una escala en la que se consideró la reducción del volumen, aclaramiento del color y mejoría de los síntomas del HI, además del grado de satisfacción paterna. Se registró el momento de respuesta inicial y máxima, efectos secundarios y secuelas.
ResultadosSe trataron 20 casos de HI (17 niñas y 3 niños). Las localizaciones predominantes fueron: periorbitaria (20%), nariz (15%), cuello/nuca (15%) y tronco (15%). La mayoría HI fueron focales y en fase proliferativa (90%). El tratamiento se inició entre 2 y 19 meses, siendo el principal motivo para empezarlo el rápido crecimiento (50%). El inicio de respuesta se observó en el 70% de los casos a los 5 días y en solo 2 tardíamente (más de un mes). El pico máximo de respuesta se obtuvo a los 3 meses. En el 55% de casos la respuesta fue excelente, buena en el 35%, mínima en el 10% y en ninguno nula. Fueron factores predictores de respuesta el HI focal, la fase proliferativa, la localización periorbitaria y la ulceración. No hemos constatado efectos adversos importantes.
ConclusiónHemos comprobado la efectividad clínica del PO en la reducción de los HI, pero no su completa desaparición al concluir el tratamiento, persistiendo parte de su volumen, color, telangiectasias o cicatrices. El PO ha resultado seguro bajo control ambulatorio.
Until recently, infantile hemangiomas that were growing rapidly or were expected to cause significant functional impairment or aesthetic defects were treated with high doses of oral corticosteroids with the intention of inducing regression.1 After the beneficial effects of propranolol on infantile hemangiomas were first reported,2 this drug soon became more widely used and numerous publications have appeared since then. Some authors have presented individual cases, analyzed case series, or provided reviews of those reports3–13; others have described multicenter studies.14,15 One study compared the effect of propranolol to that of oral corticosteroids.16 This literature seeks to provide a basis for establishing a safety and efficacy profile for this novel use of propranolol by shedding light on possible risks and benefits and generally guiding us toward appropriate protocols for treating and monitoring patients.
Propranolol is well known in pediatric cardiology, where it is used to treat a variety of heart conditions, but it is a novel drug in the treatment of infantile hemangiomas. Reports of clinical applications in various settings may be highly useful in helping us optimize management for the safe treatment of infants with these tumors. We therefore present a prospective study of 20 cases of infantile hemangioma treated with propranolol in order to analyze the efficacy and safety of an outpatient regimen.
Materials and MethodsAll infants brought to our hospital's pediatric dermatology clinic with hemangiomas between October 2008 and March 2011 were studied prospectively. The patients were followed until August 2011. We kept records on type of hemangioma, phase, location and distribution, size, color, and the presence or not of ulceration or other complications. In cases of head and neck hemangiomas with a segmental distribution or spanning the midline, imaging studies (nuclear magnetic resonance, nuclear magnetic resonance angiography, or ultrasound) and cardiac function and eye evaluations were ordered. Infants with periorbital hemangiomas were also examined by the ophthalmologist. Focal, deep, or mixed lesions and large hemangiomas (>5cm) were studied by Doppler ultrasound. Infants with multiple hemangiomas (5 or more) also underwent a liver ultrasound. Thyroid stimulating hormone level was determined in infants with multiple or large hemangiomas.
Patient SelectionInfants were candidates for propranolol therapy if they had hemangiomas in the proliferative phase and some type of complication (such as a compromised vital structure, ulceration, or bleeding) or if a hemangioma was growing rapidly and could be expected to cause functional impairment or mar the child's appearance. In hemangiomas that had passed the proliferative phase, presurgical use of propranolol was considered in case the drug might prove beneficial in reducing the volume of the residual tumor or if the location made removal difficult. Infants with PHACE syndrome (agenesis, hypoplasia, and tortuosity of large cerebral vessels), heart conditions requiring treatment, or a history of asthma or hypoglycemia were excluded. After the treatment was explained to the infant's parents, they were asked to give their written informed consent.
Pretreatment EvaluationAll infants underwent cardiac function evaluation, including an electrocardiogram and an echocardiogram. Weight and vital constants (blood pressure and heart rate) were checked. A complete blood work-up was ordered.
Preparation of the DrugThe pure drug was diluted in a sucrose-containing syrup in the pharmacy department of our hospital; a mixture was prepared monthly so that stabilizers or other additives would not have to be used. The concentration was increased as the infant gained weight so that larger volumes of the solution did not have to be administered at each dose. In all cases the total daily dosage was 2mg/kg in 3 doses administered by syringe into the infant's mouth.
At the end of treatment, the child was weaned from the drug by gradually reducing the daily dosage (to 1mg/kg for 15 days and then 0.5mg/kg for 15 days).
Monitoring During TreatmentPatients were checked 5hours after the first dose, 15days later and 15days after that, after which monthly check-ups were scheduled. Before each visit with the physician, the infant's blood pressure, heart rate, blood sugar, and weight were recorded in the pediatric day hospital. The findings were compared to values recorded at previous visits and to age- and sex-adjusted percentile curves on growth charts. Parents’ reports of changes in lesion color, temperature, size or consistency were recorded, as were adverse events. The infant was then examined and hemangioma size, color, consistency and changes in functional characteristics were noted. Photographs were taken at each visit. We noted the time of initial response and peak effect (point after which no further response of the hemangioma was observed). At the end of treatment, we recorded the presence and type of residual signs.
Assessment of Overall Response to TreatmentAt each follow-up visit, response to therapy was categorized as excellent, good, minimal, or none by a dermatologist and a pediatrician, who had to come to full agreement on an assessment. Degree of parent satisfaction was also recorded on a scale of 0 to 5, where 0 indicated complete dissatisfaction and 5 total satisfaction. All criteria defining each of the 4 global response categories used by the physicians had to be met. An excellent response was characterized by a) an estimated reduction of more than 50% of the volume of the hemangioma; b) 50% clearing of the lesion (color fading); c) recovery of functional deficit or resolution of bleeding or ulceration; d) minimal residual effects (erythema, telangiectasia, or scarring); and e) a parent satisfaction score between 4 and 5. A good response was characterized by a) a reduction in hemangioma volume of 25% to 50%; b) 25% to 50% clearing; c) recovery of functional deficit or resolution of bleeding or ulceration; d) residual effects (either minimal or evident); and e) a parent satisfaction score between 3 and 4. A minimal response was characterized by a) a reduction of less than 25% in the volume of the hemangioma; b) lack of functional recovery or resolution of bleeding or ulceration; and c) a parent satisfaction score of less than 3. Lack of response to therapy was defined by a) continued growth of the hemangioma or b) withdrawal of the drug because of adverse events. The assessments shown in Table 1 correspond to the last evaluation at the end of treatment (when possible) or at the last treatment during the study period (for patients still on propranolol).
Assessment of Overall Effect of Propranolol Treatment for Infantile Hemangiomas.
| Case No. | Volume Reduction | Clearing, or Color Fading | Resolution of Functional Impairment, Ulceration, or Bleeding | Residual Signs | Parent Satisfaction | Overall Response |
| 1 | >50% | >50% | Yes | Telangiectasia, hemosiderotic staining | 5 | Excellent |
| 2 | >50% | >50% | Yes | Superficial scarring | 5 | Excellent |
| 3 | >50% | >50% | Yes | Telangiectasia | 5 | Excellent |
| 4 | 25%–50% | <25% | No | 4 | Good | |
| 5 | <25% | <25% | 3 | Minimal | ||
| 6 | >50% | >50% | Yes | Bluish coloring | 5 | Excellent |
| 7 | 25%–50% | 25%–50% | Yes | 3 | Good | |
| 8 | >50% | >50% | Yes | Telangiectasia | 5 | Excellent |
| 9 | >50% | >50% | Yes | Scarring, moderate thickness | 5 | Excellent |
| 10 | >50% | >50% | Yes | Superficial scarring | 5 | Excellent |
| 11 | >50% | >50% | Yes | Pink color | 5 | Excellent |
| 12 | <25% | 25%–50% | No | 4 | Minimal | |
| 13 | 25%–50% | 25%–50% | Yes | 5 | Good | |
| 14 | >50% | >50% | Yes | Scarring | 5 | Excellent |
| 15 | 25%–50% | 25%–50% | Yes | 4 | Good | |
| 16 | >50% | >50% | Yes | Bluish color | 5 | Excellent |
| 17 | >50% | >50% | Yes | Scarring | 5 | Excellent |
| 18 | <25% | 25%–50% | No | 4 | Good | |
| 19 | <25% | 25%–50% | Yes | 4 | Good | |
| 20 | 25%–50% | >50% | Yes | Telangiectasia | 4 | Good |
Qualitative variables are expressed as number and percentage and quantitative variables as mean (SD). The Kolmogorov-Smirnov test was used to check whether quantitative variables were normally distributed. Qualitative variables were compared with a χ2 test and quantitative and qualitative variables were compared with a t test after Levene correction. Statistical significance was set at a value of P≤.05. Data were recorded and processed with the SPSS statistical package, version 17.0.
ResultsPatient and Hemangioma CharacteristicsA total of 45 infants with hemangiomas were evaluated during the study. Oral propranolol therapy was proposed for 23; treatment was rejected by the parents of 3 infants and accepted by the parents of 20 (17 girls, 3 boys). Thus, 44% of the infants with hemangiomas who were evaluated were treated with oral propranolol. The characteristics of these patients and their lesions are shown in Tables 2 and 3 and Figs. 1–5.
Individual Patient and Hemangioma Characteristics.
Abbreviations: ASD, atrial septal defect; DA, ductus arteriosus; DUS, Doppler ultrasound; F, female; M, male; NMR, nuclear magnetic resonance; OC, oral corticosteroids; PFO, patent foramen ovale; Prolif, proliferative; US, ultrasound; Superf, superficial.
Study Population Characteristics.a
| Sex | |
| Female | 17 (85) |
| Male | 3 (15) |
| Age, mean | 5.2 mo |
| Age, range | 2–19 mo |
| Age, at start of treatment | |
| <6 mo | 15 (75) |
| ≥6 mo | 5 (25) |
| Distribution of the hemangioma | |
| Segmental | 2 (10) |
| Focal | 17 (85) |
| Multifocal | 1 (5) |
| Type of hemangioma | |
| Superficial | 5 (25) |
| Mixed | 14 (65) |
| Deep | 1 (5) |
| Growth phase | |
| Proliferative | 18 (90) |
| Stabilized | 2 (10) |
| Prior treatments | |
| Oral corticosteroids | 5 (25) |
| Location | |
| Periorbital area | 4 (20) |
| Nose | 3 (15) |
| Trunk | 3 (15) |
| Neck or nape of the neck | 3 (15) |
| Other | 7 (35) |
| Reason for treatment with propranolol | |
| Rapid growth | 10 (50) |
| Ulceration | 4 (20) |
| Compromised eye opening | 4 (20) |
| Prevention of residual defects or deformities | 2 (10) |
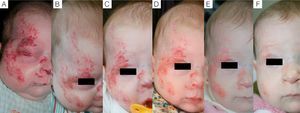
Case 1. A, Segmental hemangioma on the face, occluding the eye before treatment with propranolol. B, After 5 days of treatment the eye is beginning to open. C, At 12 days the eye is nearly open and the hemangioma is clearing. D, After 3 months of treatment. E, At 6 months. F, At 11 months (1 month after end of treatment).
The response to the drug was excellent for 55% of the treated infants, good for 35%, and minimal for 10%. Complete lack of response was never observed (Table 1).
Factors Predictive of ResponseThe variables sex, hemangioma type, and location were not significantly associated with degree of response, although it was noteworthy that an excellent response was achieved in 75% of the cases of periorbital hemangioma. We also noted that 80% of the hemangiomas with an excellent response were focal lesions and all were superficial or mixed types and in the proliferative phase. However, the associations between effect and these characteristics were not statistically significant. Another nonsignificant association observed was that 80% of the excellent responses were in infants who began treatment before the age of 6 months. In all cases in which propranolol treatment was indicated because the hemangioma was ulcerated, the response was excellent or good in this series. In patients prescribed propranolol because of rapid growth of the tumor, the outcome was good for 55.6% and excellent for 33.3%. Fifty-five percent of the patients with an excellent result had received another treatment before starting propranolol.
On exploring relationships between factors, we noted that 80% of patients who started treatment before 6 months of age began to respond within 2 to 3 days and response peaked by 2 months of therapy; the associations were not statistically significant, however. Onset of response was rapid in 75% of the periorbital hemangiomas and in all the facial hemangiomas. In 66.7% of nasal hemangiomas, however, onset of effect came later (after 20-35 days). Superficial hemangiomas and those in the proliferative phase also responded quickly (in 92.9% of cases for both characteristics).
DiscussionSince Leauté-Labrezze and coworkers2 first reported using propranolol for severe hemangiomas of infancy in 2008, many reports and other publications have attested to the benefits of this treatment,3,5,6,10,11,13–15 although few authors have analyzed more than 20 patients and hardly any have published prospective studies.6 Among recent Spanish publications, we single out those of Bagazgoitia and coworkers,14 who reported outcomes for 71 patients based on a multicenter retrospective design, and Bernabeu and coworkers,3 who provided a descriptive analysis of 28 cases. Our prospective study of a cohort of 20 infants aimed to evaluate the overall effect and safety of the outpatient treatment regimen we used and to explore factors that might influence the response of hemangiomas to propranolol.
The characteristics of our patients were similar to those generally described for infants with hemangiomas17 and are consistent with the patient profiles in other case series.14 Thus, most of our patients were females, and most of the vascular tumors were on the head or neck and were of focal distribution, superficial, and in the proliferative phase on evaluation. Treatment usually started when the infant was 2 to 3 months old; only 2 infant girls were older than 12 months of age when we started them on propranolol.
Most of our patients had not been treated previously, although 5 infants had been given corticosteroids, with no response. They were weaned from that medication.
Some of the infants in our series had several complications that would justify treatment, but we recorded only the principal reason for prescribing the drug. The largest group of patients (50%) were included in the interest of preventing nearby structures from becoming compromised by a rapidly growing hemangioma; risk of vital structural compromise was especially relevant in 4 infants with periocular involvement that interfered with eye opening (20%). Our inclusion of these infants is consistent with the current practice of treating high-risk hemangiomas with propranolol.18 Ulceration was the second most frequent reason for prescribing propranolol in this series.
Pretreatment cardiac assessment was unremarkable for most of our patients, but we did identify 2 infants with atrial septal defects and 1 infant with persistent ductus arteriosus. The cardiologist indicated that these findings did not rule out treatment with propranolol, however. Findings of nuclear magnetic resonance imaging and the ophthalmologist's evaluation were negative except for eye closure in some cases and retinal blood vessel tortuosity in 1 infant.
The daily dosage of 2mg/kg we used in this study is generally considered to provide a sufficient amount of the drug. Given the pharmacokinetics of propranolol, 3 doses per day are recommended. Some authors, however, have prescribed regimens of 3mg/kg/d,7 while others have started therapy with lower dosages and gradually increased the amount based on periodic evaluations.15,19 The dosage has even been doubled (to 4mg/kg/d) on observation of no response.3,15 It is important to point out that no oral propranolol preparation is commercially available for infants. An extemporaneously prepared formulation is therefore required. Our hospital's pharmacy department prepared the formulations for this study, so the concentration could be adjusted as the infant grew, ensuring that parents could give the correct dosage easily. The pharmacists used a sucrose-containing syrup with no added stabilizing agents, thus avoiding substances that might have triggered an allergic reaction or been toxic for such young patients. However, the best excipient for oral administration of this drug to infants has not yet been determined, and we note that incipient caries have been reported in relation to sucrose-containing formulations of propranolol.20 It is not known whether this β-blocker's effect of reducing salivation might also contribute to the development of caries.
There is clear consensus that cardiac performance should be evaluated before propranolol is started.12,14,16 A history of bronchial hyperreactivity is an exclusion criterion and blood sugar levels should be monitored at follow-up visits along with blood pressure and heart rate. Debate centers on the best way to monitor the infant taking propranolol. Some believe that the possibility of hypotension and bradycardia, particularly when large-volume or multiple hemangiomas are present, puts cardiac output at risk. Another concern that has been expressed is the risk of hypoglycemia,21 because of the possibility of permanent neurologic damage associated with this complication.6,21 These possible effects have led some to hospitalize the infant for 48hours and to start treatment with 0.16mg/kg/d and gradually increase the dosage to 2mg/kg/d if blood sugar levels and vital constants remain normal.9,19 However, long experience with large doses of propranolol in very young infants with congenital heart disease would not suggest that such an approach is necessary except during the first week of life, when the fragile neonatal condition would argue against prescribing this drug. Propranolol has been found safe even for infants with very low birthweights.22 Rare adverse events such as diarrhea23 or hyperkalemia24 have been described, but most series have observed few adverse effects, which have been of little clinical importance. Nonetheless, as this medication becomes more widely used, practitioners are usually advised to proceed with caution. We chose to treat our patients on an outpatient basis, as have other authors.5,8 We did not detect variations in blood sugar levels, such as hypoglycemia during treatment. In fact, this potential adverse event can be prevented by administering the drug with meals, avoiding starting treatment in infants less than 1 month old, and not prescribing concurrent treatment with oral corticosteroids, which can increase the risk of low blood sugar.21 It is advisable to educate parents on the possible signs of hypoglycemia so that they can take their child to a hospital if necessary.
Blood pressure—both systolic and diastolic—and heart rate stayed within normal age- and sex-adjusted ranges at all check-ups in all cases. An infant's blood pressure should be taken by a properly trained staff member to ensure that readings are accurate. The rate of weight gain was also within normal ranges for all our patients. An infant who was accidentally given a dose that was 8-fold higher than prescribed developed no problems.25 The risk that propranolol might trigger an asthma attack must be considered and treatment should be avoided or interrupted during episodes of bronchiolitis, as occurred in one of our patients. Propranolol would be formally contraindicated in cases of well-established asthma, when continuous use of bronchodilators would be required. The fact that half our patients with an excellent response to therapy had previously been treated with oral corticosteroids reflects the severity of the hemangiomas in our series.
We would like to emphasize that the outpatient regimen we used was effective and satisfactory and, as some authors have suggested previously, this approach might lower the hospitalization-related costs of this therapy.5
The effectiveness of the treatment regimen we used was excellent overall, and in no case did we observe a lack of response. Although the response evaluation system we used might have limitations, we can say that our observations are consistent with those reported from other studies that concluded that propranolol is effective for treating infantile hemangiomas. It is important to emphasize that no consensus has emerged on the best way to measure response criteria; thus, studies differ with regard to how many assessors are consulted (as many as 5 opinions have been recorded) or how color, size and other criteria are weighted.3,10,14 Our assessment system was similar to that of Bayleis and coworkers,26 although we required that 2 experts come to complete agreement and we also included parent satisfaction. We think it is important to take the parents’ perspective into consideration, although previous studies have not done so. Parent satisfaction is a key element in adherence to treatment and in cooperation with adequate follow-up. Perhaps consensus on this would be a goal experts might consider for the future.
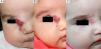
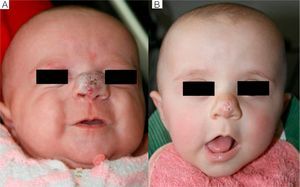
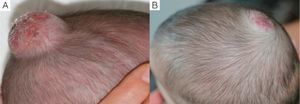
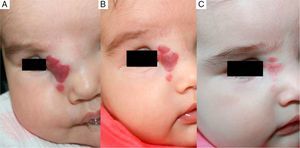
We observed that in all cases of periorbital hemangioma, treatment led to rapid improvement in eye opening, consistent with reports from earlier studies11,14 and suggesting a good prognosis for vision in the affected eye. Case 1 (Fig. 1) was exemplary: the ophthalmologist detected retinal blood vessel tortuosity on initial examination of that infant but vessels were nearly normal on later evaluation. Other authors have also observed improvements in such eye defects or vision problems as microphthalmia or astigmatism.27 Treatment with propranolol in such cases would be quite clearly indicated, therefore, and this drug is in fact now considered a first-line treatment for hemangiomas that put the eye at risk.13 Nonetheless, we note that clinical response was minimal in an infant in our series who had an eyelid hemangioma and for whom treatment started late, at 17 months. The response of nasal hemangiomas in our series was very variable. In only 1 case was the outcome considered excellent (Fig. 2), whereas response was excellent for a scalp hemangioma (Fig. 3). None of the infants we treated had respiratory tract involvement. One infant in our series presented hemangiomatosis, although no visceral organs were affected. We decided to start treatment when we saw that the number of lesions had reached 23, after increasing by 2 every day. With treatment, no new hemangiomas presented and the already evident lesions decreased in number. In a previously reported case, diffuse neonatal hemangiomatosis associated with lesions on visceral organs responded to propranolol.28
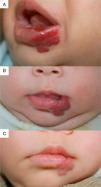
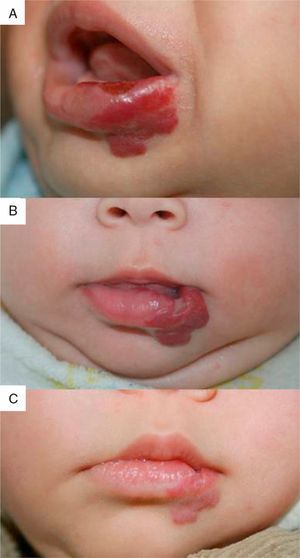
We observed the rapid resolution of ulceration in all cases where that complication was present, regardless of whether the hemangioma was on the lip (Fig. 4), elsewhere on the face, or on the back or arm. Oral corticosteroids had been given to all the infants with ulcerated hemangiomas. As those drugs were quickly withdrawn so that propranolol therapy could begin, we conclude that corticosteroid treatment did not have a decisive effect on the final result obtained. The time until appearance of scarring, which would indicate the moment of peak effect, ranged from 2 to 6 months (mean, 4.2 months). Some authors who have reported excellent results recommend that propranolol be considered a first-line option for ulcerated infantile hemangiomas.9 However, others have observed inadequate resolution of ulceration with propranolol treatment in most cases,3 so the suggestion that this drug is a clear alternative to corticosteroids or laser therapy29 remains to be demonstrated.
We would like to emphasize how quickly improvement could be seen in a high percentage of cases. Some parents reported changes (fading of color or softening of the lesion) only 2 days after treatment began, and we observed such changes at the 5-day check-up. When the eye was occluded, opening was achieved within a week of treatment (Fig. 1). The peak effect most often occurred before 5 months of therapy in our series, and in only 3 cases did the first sign of response come late (at 1 month). These observations are consistent with the literature regarding rapid onset of effect3,11,12 and time required to reach the peak response.14 When onset of effect came early in our patients, the overall outcome was good or excellent. When response came later, however, the overall effect was less complete. Such cases might be considered partial responses and possibly dosage increases should be considered early.
Most infants in our series were treated for 6 to 7 months; 1 infant was treated for 14 months and 3 were still on treatment when the study ended. The endpoint of treatment is an important issue because propranolol is known to have an effect not only in the proliferative phase but also later and it is not clear when treatment should cease.30 Following practices that tend to be accepted at present, we continued treatment for 6 months or until the end of the proliferative phase to guard against regrowth. In 2 cases (patient numbers 1 and 10), regrowth occurred when the dosage was lowered and in 1 case (number 8) regrowth started after withdrawal of the drug altogether. Treatment had to be reinstated in those cases. Propranolol also acts after the proliferative phase and if treatment must be restarted it has been seen to act with the same efficacy.4
Most side effects of treatment in our patients were nonspecific and of little clinical significance, although events described in the literature have included sleep disturbances, listlessness, restlessness, loss of appetite, or cold fingers and toes. We did not observe any of the more serious adverse effects, such as hypoglycemia, hypotension, diarrhea, hypokalemia, or respiratory difficulty. The episode of bronchiolitis in our series did not prevent the infant from continuing propranolol treatment once the infection had been brought under control.
Propranolol treatment arrests the progression of growth in the proliferative phase and accelerates the involution phase but does not achieve complete disappearance of the hemangioma in most cases. However, therapy does lead to significant improvement, in the form of reduced volume and clearing or fading of color. Residual effects, such as pigmentation changes, telangiectasia, or scarring are seen even in cases in which response has been classified as excellent. The presence of hemosiderotic coloring at the edges of a lesion may be transient, attributable to blood deposited as vessels are destroyed. Once the peak effect has been reached, further improvement is unlikely to derive from indefinitely prolonging treatment of the hemangioma.
In conclusion, even considering the limitations of this small case series, we feel that propranolol is an effective, fast-acting drug for treating infantile hemangioma. However, as therapy does not achieve complete resolution, pigmentation changes, telangiectasia, or superficial scarring will remain. We also conclude that this use of propranolol is safe, does not cause significant side effects, and can be monitored on an outpatient basis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Betlloch-Mas I, et al. Tratamiento de hemangiomas infantiles con propranolol en régimen de control ambulatorio. Estudio prospectivo. Actas Dermosifiliogr. 2012;103:806-15.