Psoriasis is a chronic inflammatory disease associated with multiple comorbidities, particularly in patients with arthritis or more severe forms of the disease. The link between all these comorbidities is probably systemic inflammation. Several recent studies have indicated that patients with psoriasis may be at an increased risk of pathologic fractures and osteoporosis. Current guidelines on comorbidities in psoriasis do not recommend assessment of bone health. In this article, we review the available evidence on the association between psoriasis and osteoporosis. We first examine the concept of osteoporosis and the role of vitaminD in bone health and then propose an algorithm for managing and treating this condition in patients with psoriasis.
La psoriasis es un proceso inflamatorio crónico que se ha asociado con múltiples comorbilidades, especialmente las formas más graves y asociadas a artritis. El estado de inflamación sistémica es, probablemente, la conexión entre todas estas enfermedades concomitantes. Algunos trabajos recientes indican que los pacientes con psoriasis pueden tener mayor riesgo de fracturas patológicas y osteoporosis. Las guías actuales de abordaje de las comorbilidades de la psoriasis no incluyen valoración de la salud del hueso. Por eso, en este artículo nos proponemos revisar la evidencia disponible sobre la relación entre psoriasis y osteoporosis. Repasaremos primero el concepto de osteoporosis, abordaremos también el papel de la vitaminaD en el hueso y, por último, proponemos un algoritmo de manejo y tratamiento de la osteoporosis en el paciente con psoriasis.
Psoriasis is a cutaneous inflammatory disease characterized by chronic and recurrent erythematous scaly plaques and affecting 0.1% to 2.9% of the world population1,2; in Spain, the estimated prevalence is 2.3%.3 Between 7% and 42% of patients with psoriasis present inflammatory arthritis.4 Psoriasis is associated with several comorbidities in addition to joint disease, including cardiovascular disease, hypertension, obesity, diabetes, dyslipidemia, and fatty liver, as well as a higher risk of death.5 Systemic inflammation is probably the connection between all these concomitant diseases. Some of these comorbidities have been reviewed in previous articles.6–8
Recently, other entities, such as osteoporosis (OP), have also been considered as comorbidities of psoriasis.9 Some studies indicate that patients with psoriasis have a higher risk of pathological fractures,10,11 as is the case with other inflammatory diseases, such as rheumatoid arthritis and atopic dermatitis, in which the incidence of OP is 4.72-fold higher than in individuals without the disease.12 Three potential mechanisms may explain this association between inflammatory diseases and accelerated bone loss. First, there is a direct effect of some cytokines and proinflammatory molecules (for example, IL-1, IL-6, IL-11, IL-15, IL-17, RANKL, and TNF-α) in bone, which might accelerate bone loss13; second, some treatments used in inflammatory diseases might contribute to bone loss, for example, corticosteroids, particularly when used systemically12; and third, immobility and lack of exercise (which may affect patients with chronic musculoskeletal diseases) also increase bone resorption.
Current guidelines on management of comorbidities of psoriasis do not include bone health.14,15 For this reason, in the present article, we will review the evidence available on the relationship between psoriasis and OP. To introduce the topic, we will first review the concept of OP, then consider the role of vitamin D in bone physiology, and finally, propose a management and treatment algorithm for OP in patients with psoriasis.
OsteoporosisConceptOP is defined as a generalized disease of the skeleton that is characterized by low bone density and altered microarchitecture leading to increased bone fragility and, as a result, increased risk of fracture. Given the impact this has on quality of life, morbidity and mortality, and socioeconomic factors, it is important to take preventive measures. Therefore, OP is currently considered as an important public health issue.16
The current concept of OP, understood to be decreased bone resistance, goes beyond the mere concept of bone mineral density (BMD) to also encompass qualitative aspects of bone quality. BMD can be measured by densitometry and is closely associated with the presence of fractures, given the risk of fracture increases by a factor of 2 for every SD decrease in BMD as measured by dual-energy X-ray absorptiometry (DXA). BMD is thus currently considered one of several risk factors which may help when assessing the risk of fracture in a patient. Fracture prevention is the ultimate objective of our clinical approach.
Bone DensitometryIn 1994, the World Health Organization17 established a classification for BMD for diagnosis of OP in postmenopausal women, in which 4 categories were established according to the DXA findings in a study of the spine or femoral neck (Table 1). The scale used for measurement of bone mass (T-score scale) requires comparison of individual's bone mass with that of healthy young women (30-35years), rather than the Z-score, in which the comparison is established as a measurement of bone mass in women of the same age.
Diagnostic Categories of Osteoporosis (World Health Organization 1994).
| Category | Definition | Predictive Value of Fracture |
|---|---|---|
| Normal | T-score between +1 and 1 | Low risk of fracture |
| Osteopenia | T-score between −1 and −2.5 | Moderate risk of fracture |
| Osteoporosis | T-score < –2.5 | High risk of fracture |
| Severe osteoporosis | T-score<–1.5 plus fractures |
These criteria are not applicable to premenopausal women or to men under 50 years of age, as the relationship between decreased BMD and risk of fracture is less well defined in these populations than in postmenopausal women. In these cases, the Z-score should be used, in which a decrease of −2.0 SD is below the expected range for the age of the patient.18
However, studies have shown that approximately half of all hip fractures occur in women without OP identified by densitometry,19 and so one of the limitations of this technique is its low sensitivity for identifying subjects who will experience fracture.
Clinical Risk Factors for OsteoporosisDensitometry contributes less than a third to the prediction of fracture risk. Age and clinical risk factors are important for predicting fractures. The higher the number of risk factors in the same individual, the greater the future risk of experiencing a fracture.
Clinical risk factors that have been demonstrated to be the most consistent in different studies20 in their association with risk of fracture are as follows:
- –
Advanced age (relative risk [RR]>2). Age is one of the main risk factors for developing a fracture. Age also influences the importance of decreased bone density; thus, in younger individuals (50-60years), the decrease in BMD represents a much lower risk of fracture than in older individuals.
- –
Family history (parents and siblings) of hip fracture (RR>2).
- –
Personal history of previous fracture (peripheral and/or vertebral) at ages greater than 50 years (RR>2). This includes radiographic or morphometric fracture.
- –
Body mass index ≤19kg/m2 (RR>2).
- –
Female sex (RR>1 and >2).
- –
Smoking habit (RR> 1 and> 2).
- –
Alcohol use (daily consumption ≥30g/dL) (RR> 1 and >2).
Other relevant clinical risk factors and those included in the risk scales are associated with medication use (for example, corticosteroids, anticonvulsants) or the presence of diseases that may lead to secondary OP (for example, rheumatoid arthritis, type1 diabetes, anorexia nervosa, and hypogonadism).
Other factors associated with the development of fractures because of a higher risk of falls are postural instability, having had 2 or more falls in the past year, inability to get up from a chair, and loss of visual capability.
Conventional RadiographyConventional radiography has not been shown to be a sensitive or specific method for assessing changes in bone density, but the technique is necessary to check for the presence of fractures. It is usually enough to perform a lateral radiograph of the dorsal spine (centered on D7) and the lumbar spine (centered on L2), although, additionally, an anteroposterior view may also be appropriate.
Conventional radiography is considered in patients with spinal pain, those with a decrease in stature (documented loss of 2cm height or 4-6cm compared with height recorded in youth), and in those with OP densitometric values (T score index <−2.5T), because vertebral fractures are often asymptomatic and may have gone unnoticed by the patient; detection of their presence is important both for follow-up of the patients and for accurate assessment of the risk of successive fractures.
Assessment of Fracture RiskMore than a decade ago, in clinical practice, densitometry defined osteoporotic disease and was used for decision making in the prevention of osteoporotic fractures. Today, it is recommended to assess the risk of fracture. Different risk assessment scales have been developed and these represent essential support for clinical decision making. They estimate the absolute risk of fracture in the following years (usually they consider the risk at 10 years), based on age and clinical risk factors that have proved to be most consistent. Different models have been developed to assess the probability of fracture based on the combination of several independent risk factors. One of the most widely applied is the FRAX model, which is extensively used internationally. It was introduced in 2008 by a group of experts led by Kanis21 and supported by the World Health Organization. It is an algorithm for calculating the fracture risk available from the Internet and is designed to determine the absolute risk at 10 years of hip fracture and mayor clinical osteoporotic fracture (clinical vertebral, forearm, proximal humerus) in patients between 40 and 90 years according to whether the most predictive risk factors are present, with or without the inclusion of BMD and is specific by country (Fig. 1). In the general population, active therapeutic intervention is advised for a risk assessment above a certain threshold (> 10% for major fractures and/or > 3% for hip fractures, according to country and author). Given that FRAX appears to underestimate the risk of fracture in the Spanish cohort,22 new studies have reassessed its utility in the Spanish population,23 and treatment is now proposed in patients with >7.5% risk of major osteoporotic fracture at 10 years and with risk of >3% for hip fracture.
However, it should be remembered that this is a tool for guidance only, given that it is subject to major limitations. For example, it does not consider the number of prior fractures or corticosteroid dose, it does not differentiate between vertebral fracture and other fractures, and it does not consider falls. Furthermore, it has not been specifically studied in patients with psoriasis.
Osteoporosis and PsoriasisPathogenic Mechanisms Connecting Psoriasis and OsteoporosisIn physiological conditions, there is an equilibrium between bone formation and resorption to ensure skeletal homeostasis. In pathological conditions, this equilibrium is shifted towards osteoclast-mediated bone resorption. Metabolic activation of osteoclasts to enhance the capacity for bone resorption requires complex signaling between cells of osteoclast lineage, mesenchymal cells, and lymphocytes.24,25 These interactions are controlled by several cytokines and by the receptor activator of nuclear factor κB (NF-κB) ligand, known as RANKL. RANKL is produced by a range of cells, including some immune cells, cells in the vascular wall, and osteoblasts. This factor belongs to the tumor necrosis factor (TNF) family. When RANKL binds to its receptor (RANK), present in the membrane of osteoclast precursors, it induces a series of signals that promote differentiation of these precursors and the formation of osteoclasts.25 In addition to RANKL, osteoblasts produce osteoprotegerin (OPG), which is a RANKL inhibitor. This is a soluble protein that binds to RANKL and prevents its interaction with its RANK receptor. This OPG-RANKL system is essential in the process known as osteoclastogenesis.
In an inflammatory environment, T cells produce RANKL, which stimulates osteoclast-mediated bone resorption. The cytokines IL-1 and TNF can enhance the effects of RANKL, favoring bone resorption through direct stimulation of osteoclast precursors and mature osteoclasts. Some proinflammatory cytokines, such as IL17, are also known to be associated with osteoclastic bone resorption in other inflammatory diseases such as rheumatoid arthritis.13
TNF acts as a trigger for osteoclastogenesis through expression of a series of transcription factors such as NF-κB, which is critical for the process. The effect of TNF on other bone cells, such as osteoblasts and osteocytes, is less well established. Models of rheumatoid arthritis have found a decrease in osteoblastic activity through inhibition of a Wnt/b-catenin signaling pathway.26
The humoral and cell mechanisms proposed to explain bone loss has points in common with psoriasis pathogenesis. TNF and IL-17 are relevant cytokines in the pathogenesis of psoriasis (as they are in OP), and they are also considered possible therapeutic targets to suppress the hyperreactivity of the immune system and restore the equilibrium between bone resorption and formation. Treatments that control articular inflammation, such as anti-TNF agents, have beneficial effects on systemic bone remodeling in patients with rheumatoid arthritis, and this translates into increased BMD measured by DXA.27,28
Bone Risk Factors Associated with PsoriasisOther important risk factors for OP and fracture have been reported in patients with cutaneous psoriasis, such as the duration, activity, and extent of skin disease (moderate to severe psoriasis),10,29,30 and the presence of psoriatic arthritis (PsA),10,31–35 and/or ankylosing spondyloarthritis (AS).36 The changes that occur in the joints of patients with PsA are due to inflammatory synovitis. Psoriatic arthritis is primarily a form of enthesitis. Subsequently, involvement spreads to perientheseal tissues leading to synovitis and osteitis. One of the main characteristics of chronic inflammatory arthritis is destruction of cartilage and bone, which is associated with systemic OP and a higher risk of fractures due to fragility.37 Also, in AS, low bone mass has also been found, particularly in men, and the etiology has not been sufficiently clarified.38 OP can also occur due to physical inactivity and decreased mobility of the spine as a result of pain, rigidity, and ankylosis.39
In psoriasis, both sexes have a high prevalence of OP and fractures; some studies, in contrast to the general population, have found a higher prevalence in men with psoriasis.11,34,40 In a study in the Spanish population,41 patients with psoriasis showed significantly lower BMD in the spine and hips than controls. Another study reported a decrease in BMD only in patients with PsA and not in those with psoriasis, although the mean Psoriasis Area and Severity Index (PASI) was 7.8 and the disease duration was lower than in patients with PsA.42 Some studies have found significantly lower serum levels of vitamin D in patients with psoriasis and OP, and low levels are considered a risk factor in this population.43 The importance of vitamin D in psoriasis is analyzed in more detail in another section of this review.
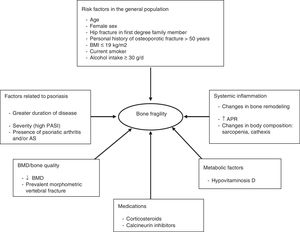
Figure 2 shows the different factors that can contribute to bone fragility in patients with psoriasis.
Table 2 summarizes studies of OP and psoriasis.
Studies of Psoriasis and Osteopenia/Osteoporosis.
| n | Osteopenia | Osteoporosis | Results | |
|---|---|---|---|---|
| Borman et al.31 | 47 with PsA | 27.5%50% | 5% | A >duration of PsA <lumbar spine and femoral BMD |
| Attia et al.32 | 50 | Patients with PsA, plus OPA>no. of joints affected> risk of OP | ||
| Balato et al.34 | 10226 with PsA | 24%46% | 5%17% | Greater osteopenia in men |
| D’Epiro et al.29 | 4319 with PsA | Duration of psoriasis>in patients with OPN/OP | ||
| Keller et al.30 | 17 507 OP52 571 controls | OR psoriasis 1.65 (95%CI: 1.42-1.94)OR severe psoriasis 1.96 (95%CI: 1.37-2.81) | Taiwan population | |
| Chandran et al.33 | Systematic review of 21 studiesPsA | Prevalence 1.4% to 68.8% | Age, female sex, postmenopausal, duration of arthritis, presence of erosions, and cumulative steroid dose associated with < BMD | |
| Solak et al.43 | 43 psoriasis vs 41 controls | 44.4% | 18.5% | >% of OPN and OP in psoriasisWomen with OP have very low vitamin D levels |
| Dreiher et al.40 | 7936 psoriasis patients vs 14 835 controls | Men 3.1% vs 1.7%, P<.001, OR 1.86 (95% CI: 1.44-2.39)Women 22.3% vs 20.2%, P<.008, OR 1.13 (95% CI: 1.03-1.25) | Adjusting for confounding factors, psoriasis was significantly associated with OP in men, adjusted OR 1.70 (95% CI: 1.31-2.19), P<.001 | |
| Kathuria et al.36 | 183 725 psoriasis 28 765 PsA | OR 2.86 (95% CI: 2.70-3.02) | OR 2.97 (95% CI: 2.89-3.06) | |
| Martinez-Lopez et al.41 | 57 psoriasis vs 61 controls | Low levels of BMD | Age risk factor, high BMI protective factor |
Abbreviations: BMD, bone mineral density; BMI, body mass index; OP, osteoporosis; OPN, osteopenia; OR, odds ratio; PsA, psoriatic arthritis.
In the case of fractures, the main consequence of OP, there are few studies in patients with psoriasis and the results are contradictory.10,11,35,36,44 Only one of the studies did not find a clear association between psoriasis and fracture risk and OP. Limitations of this study included self-reported diagnosis of psoriasis with no information on disease severity; the authors themselves recognized that certain subgroups of psoriasis, such as the most severe forms, early-onset psoriasis, or PsA might be associated with a higher risk.44Table 3 summarizes studies of pathological fractures and psoriasis.
Studies of Psoriasis and Fracture Risk.
| n | Fractures | Results | |
|---|---|---|---|
| Pedreira et al.35 | 52 women with psoriasis45 women with PsA98 controls | Greater risk in psoriasis and PsA | Recurrent falls and a longer disease duration increased the risk of fracture |
| Ogdie et al.10 | Psoriasis 158 323PsA 9788Controls 821 834 | Mild psoriasis aHR 1.07 (1.05-1.10)Severe psoriasis aHR 1.26 (1.15-1.39)PsA aHR 1.26 (1.06-1.27) | British population |
| Kathuria et al.36 | 183 725 psoriasis28 765 with PsA | Psoriasis: spinal (1.17; 1.09-1.25), pelvic (1.18; 1.06-1.31), femoral (1.68; 1.60-1.78), and tibial/fibular (1.28; 1.16-1.41) fracturesPsA: stress (2.87; 1.08-7.64), vertebral (1.45; 1.24-1.70), pelvic (1.75; 1.41-2.18), femoral (2.07; 1.85-2.32), and tibial/fibular (1.60; 1.28-2.01) fractures | US population |
| Modalsli et al.44 | 2804 self-reported psoriasis | HR adjusted for age and sex 1.03 (95% CI: 0.82-1.31) | No increase in the risk of fracture or OP (Norwegian population) |
| Paskins et al.11 | 24 219 psoriasis (802 severe)1008 PsA94 820 controls | Psoriasis: HR 1.10 (95% CI: 1.04-1.16)/aHR 1.14 (95% CI: 1.08-1.21)PsA: HR 1.26 (0.95-1.65)/aHR 1.62 (1.20-2.18) | Greater risk in men than in women and apparently greater in patients with PsAThe use of methotrexate is not associated with greater risk (HR0.91; 95% CI: 0.72-1.15) |
Abbreviations: aHR: adjusted hazard ratio; BMD, bone mineral density; HR, hazard ratio; OP, osteoporosis; PsA, psoriatic arthritis.
Other factors that may impact the association between psoriasis and OP are the drugs used in the treatment of the psoriasis (corticosteroids, methotrexate, and ciclosporin) as these may affect bone density. Those with greatest influence are oral corticosteroids when used over long periods of time (for more than 3 months at a dose equivalent to 5mg/day of prednisone or greater).
The negative effects on bone of systemic corticosteroids are well known. Continued use of high cumulative doses of topical corticosteroids could be a risk factor for OP in patients with psoriasis. Cases have been reported of multiple bone fractures due to continued use of topical corticosteroids in psoriasis.45 However, Haeck et al.46 did not find a significant association between decreased BMD and use of topical and systemic corticosteroids in patients with atopic dermatitis.
The association between retinoid use and radiographic bone abnormalities has not been demonstrated. The prevalence of diffuse vertebral hyperostosis, ligamentous calcifications, and OP in elderly individuals who do not use retinoid drugs complicates the interpretation of these findings. Although there may be a relationship between the use of these drugs and skeletal abnormalities, only a small number of patients seem to be affected after prolonged use and the effects are usually asymptomatic.47
The effect of methotrexate on BMD has not been extensively studied. In patients with rheumatoid arthritis, the use of low doses of methotrexate was not associated with bone loss in the spine or hips after 3 years of follow-up. However, in patients who were also treated with prednisone (>5g/day), methotrexate was associated with greater bone loss in the spine, suggesting an additional effect on bone loss beyond that expected from the effect of the corticosteroids alone.48 Uehara et al.49 showed in an in vitro study that methotrexate hinders bone formation by inhibiting the differentiation of osteoblast precursors. The therapeutic effect, with control of the systemic inflammation of the disease, may, however, compensate for the agent́s pootential deleterious effect on bone. Recent studies have not found a greater risk of fracture in patients with psoriasis treated with methotrexate, compared with other patients with psoriasis who had not received this drug.11
Calcineurin inhibitors (ciclosporin, tacrolimus) could interfere with osteoblast differentiation by inhibiting the calcineurin-NFAT signaling pathway. Different in vitro studies have shown contradictory results in terms of effects on bone. Overall, they appear to be associated with bone loss, but their impact on fracture is not well established. However, most of the studies have been performed in patients with solid organ transplant who have also been treated with corticosteroids, and this hinders drawing definitive conclusions.50
As mentioned previously, anti-TNF treatments have shown benefit in patients with rheumatoid arthritis in terms of systemic bone remodeling, with increased BMD as measured by DXA, due to control of chronic inflammation and improved physical activity.27,28
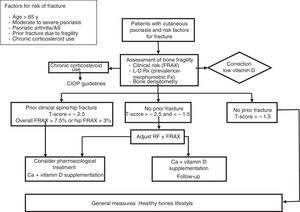
Management and Treatment of Osteoporosis and the Risk of Fracture in Patients With PsoriasisThere are no specific guidelines for the treatment of OP in psoriasis. In view of the above discussion, bone assessment is recommended in patients of both sexes with moderate to severe psoriasis, with associated PsA, and in patients in chronic treatment with oral corticosteroids, in presence of other factors mentioned that have been shown to be predictive of risk of fracture in the general population, such as age > 65 years and presence of previous fractures. In these patients, dermatologists should assess the risk of fracture through study of the clinical risk factors and using fracture risk scales (FRAX). Bone densitometry and/or dorsal and lateral lumbar radiography enable the detection of prevalent morphometric fractures and increase the probability of requiring pharmacological treatment. Patients should be assessed by specialists, such as rheumatologists, endocrinologists, and, as always, the primary care physician.
Disease control to minimize bone loss associated with systemic inflammation and ensuring that corticosteroids are given in the lowest possible dose for the shortest possible duration are essential measures. Patients in chronic treatment with corticosteroids should follow the published guidelines for the prevention and treatment of corticosteroid-induced OP.51
The main objective of OP treatment is to reduce the risk of fracture. The therapeutic approach involves pharmacological measures basically aimed at increasing bone resistance and nonpharmacological measures, with the aim of maintaining as far as possible a good state of health, decreasing the risk of falls, and minimizing their consequences.
A healthy lifestyle is the first option for preventing OP.
- –
Encourage physical activity, which can increase agility, strength, posture, and muscle balance, and reduce the risk of falls.
- –
Cover the nutritive needs with a healthy diet, which includes suitable intake of calcium (1000-1200mg/day).
- –
Sunlight as source of vitamin D. It is important to guarantee daily exposure to sunlight on the hands, face, and arms for at least 10 to 15minutes per day. The recommendations on exposure to sunlight should take into account the potential risk for dermatological lesions, remembering that use of sunscreens can reduce the effectiveness of exposure for synthesis of vitamin D.
- –
In patients with low levels of vitamin D, daily supplements with 800 IU of vitamin D are recommended. With this dose, levels between 20 and 40 ng/mL are attained in adult and elderly individuals. These are the levels that are necessary to achieve the beneficial effects on bone health and that have been found to be effective in prevention of fractures.
- –
Avoid alcohol intake and smoking
- –
Programs for fall prevention
In general, the decision when to administer pharmacological treatment is based on the assessment of absolute risk of fracture, and this is well defined by age. Treatment should be tailored to each patient, taking into account his or her particular circumstances. In Spain, all the antiosteoporotic drugs available have demonstrated efficacy to a greater or lesser extent against vertebral fracture in clinical trials with a robust methodology. Some have also demonstrated efficacy against nonvertebral fractures, including hip fractures. In general, pharmacoeconomic studies have established that antiosteoporotic drugs are cost-effective in populations with important risk factors—advanced age, low BMD, and history of previous fracture—and that bisphosphonates have a better pharmacoeconomic profile and so are considered the first-choice agent for the treatment of OP.
Pharmacological intervention is performed with therapeutic agents able to act on the 2 phases of bone remodeling. There are 2 main categories:
- –
Antiresorptive or anticathobolic drugs, which inhibit bone resorption by acting on osteoclasts or their precursors, decrease the activation rate of bone remodeling, increase BMD, and preserve bone microarchitecture. This group includes raloxifene, bazedoxifene, bisphosphonates (alendronate, risedronate, ibandronate, zoledronate), and denosumab.
- –
Anabolic agents, which act on osteoblasts or their precursors, leading to an increase in bone remodeling, with greater increase in bone formation compared with resorption, thus increasing bone mass and resistance. Among the anabolic agents, the only molecule currently available is teriparatide.
In patients in treatment with drugs for the management of OP, calcium and vitamin D supplements should be used, as efficacy data are derived from clinical trials that used the drug in association with calcium and vitamin D.
Figure 3 proposes a management and treatment algorithm for OP for patients with psoriasis.
Vitamin D and PsoriasisIt is argued that lack of vitamin D might explain the increased risk of OP in patients with psoriasis. A recent meta-analysis showed that serum calcidiol levels were decreased in patients with psoriasis compared with healthy controls.52 Furthermore, they were negatively associated with disease severity as measured by PASI. Limited exposure to sunlight or the way that patients dress to hide skin lesions could contribute to the decreased levels of vitamin D.52
Kathuria et al.36 showed that the prevalence of osteomalacia is increased in patients with psoriasis. This bone mineralization disorder usually arises as a result of an imbalance in the Ca/P ratio. In almost half the cases, the imbalance is due to altered levels of vitamin D, the main regulator of calcium uptake. With the use of the fumaric acid esters used to treat psoriasis, there have been reports of osteomalacia syndrome associated with increased urinary phosphate elimination caused by proximal tubular dysfunction (Fanconi syndrome). Women with psoriasis treated for long periods with fumarates seem to be particularly susceptible to this effect.53 Osteomalacia will usually become manifest in the form of densitometric OP and with the presence of atypical stress fractures.
But in addition to its effects on bone, vitamin D has an important role in psoriasis because of its extraosseous effects. The extensive distribution of the vitamin D receptor (VDR) and the α-1-hydroxylase enzyme (CYP27B1), the enzyme required to convert circulating calcidiol to calcitriol, enables several cell types to produce their own calcitriol if sufficiently high circulating serum calcium levels are attained. The action/regulation of hormone D is thus enhanced, not only from the endocrine point of view, but also the paracrine or autocrine one. This explains its non-calcium-based actions, regulating more than 200 genes that participate in cell differentiation and proliferation, in the secretion of different hormones, and in immune activity among other actions.54
Vitamin D acts on the immune system at several stages of the immune chain, modulating antigen recognition, blocking costimulation of certain molecules, inducing T-cell regulation towards suppressor lines, antagonizing the action of inflammatory cytokines and stimulating proinflammatory ones, and modulating monocyte and macrophage traffic. Through these actions, it regulates the cutaneous immune system, stimulates expression of antimicrobial peptides (for example, cathelicidin), and plays an important role in the regulation of dendritic cells in innate immunity.55
Another extraskeletal action of vitamin D of particular interest is its antiproliferative action. When serum levels exceed 30 ng/mL, there is increased genetic induction by calcitriol of protein synthesis, with inhibitory effects on angiogenesis and inducers of tumor cell apoptosis, the so-called proteins p21 and p27. In this way, vitamin D could regulate proliferation, differentiation, and apoptosis of keratinocytes and also regulate the permeability and integrity of the skin barrier; the beneficial effect of vitamin D analogs in the treatment of psoriasis is thus explained.53,56
Conclusions- 1.
There is a pathophysiological basis to support an association between psoriasis and OP, namely, excessive production of proinflammatory cytokines and activation of osteoclastogenesis.
- 2.
Studies of impact on BMD and increased risk of facture are still somewhat limited and contradictory. Nevertheless, overall, we believe the data support this association, which is more consistent in patients with PsA, more severe psoriasis, and longer-standing disease. The role of comorbidities and other confounding factors (influence of treatment) are awaiting clarification.
- 3.
Identification of subjects with a high risk of bone fragility is justified in patients with psoriasis according to the evidence available. It is important to collaborate with other specialists, such as rheumatologists, endocrinologists, and primary care physicians, for suitable management of these patients with a high risk of fracture.
- 4.
Well-designed studies are needed to define this potential comorbidity of psoriasis given the impact of bone fragility on morbidity and mortality.
Abbvie facilitated meetings for the participants of the group but none of their employees participated in the development and elaboration of scientific material, discussion, or the written drafts.
Please cite this article as: Muñoz-Torres M, Aguado P, Daudén E, Carrascosa JM, Rivera R. Osteoporosis y psoriasis. Actas Dermosifiliogr. 2019;110:642–652.