Acute generalized exanthematous pustulosis (AGEP) is a rare skin eruption (estimated incidence, 1 to 5 cases per million population per year1) that is usually induced by drugs. It was recently reviewed in this journal.2 AGEP consists of the rapid appearance of numerous sterile nonfollicular pustules on a background of erythematous-edematous skin and is associated with fever and leukocytosis. The eruption is self-limiting after withdrawal of the offending drug. Few cases of nystatin-induced AGEP have been published. Nystatin is an antifungal agent that is widely used throughout the world and that is usually well tolerated because of its minimal systemic absorption. In all published cases, patch testing was useful to confirm the diagnosis of this toxic dermatitis.
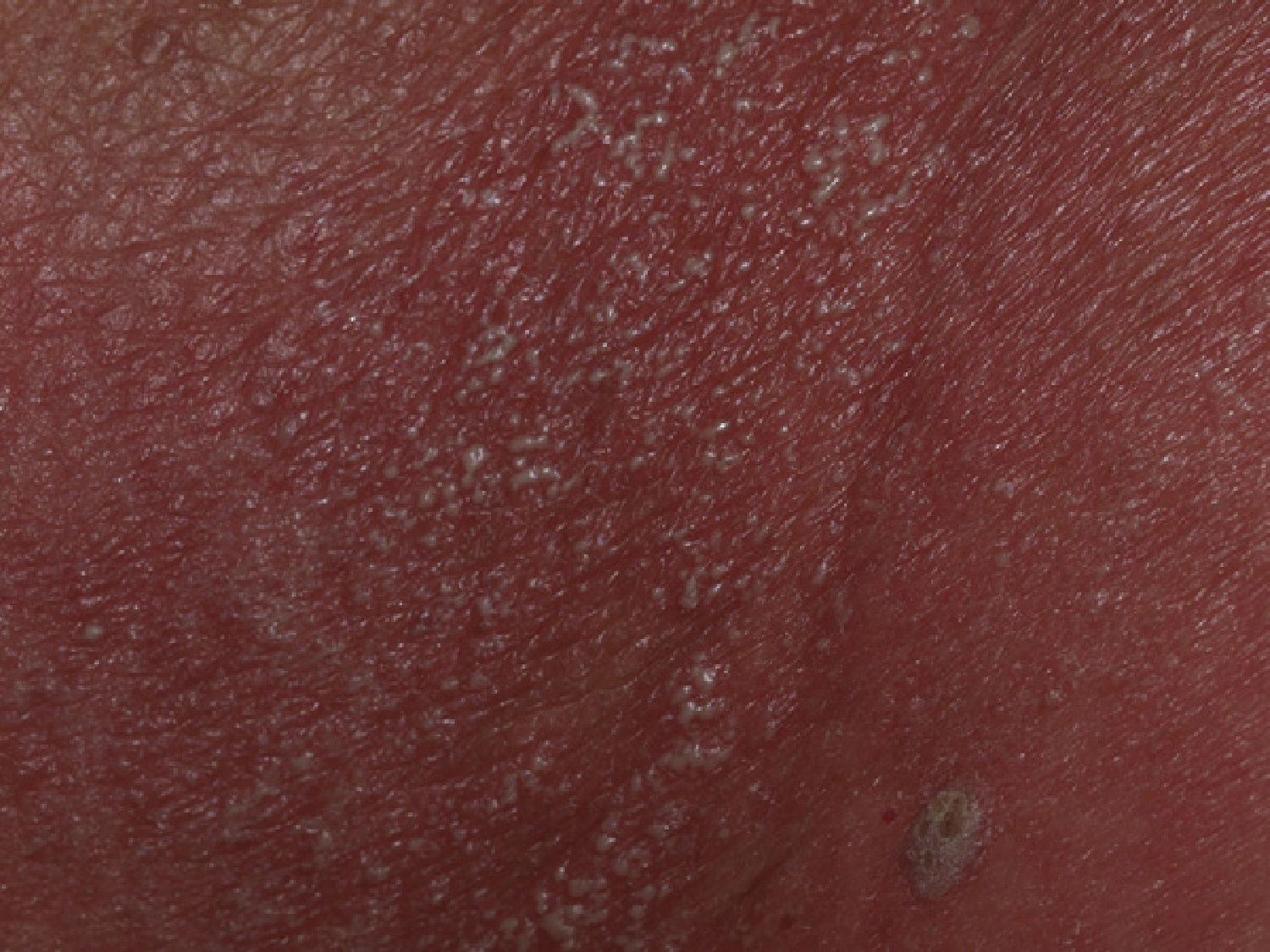
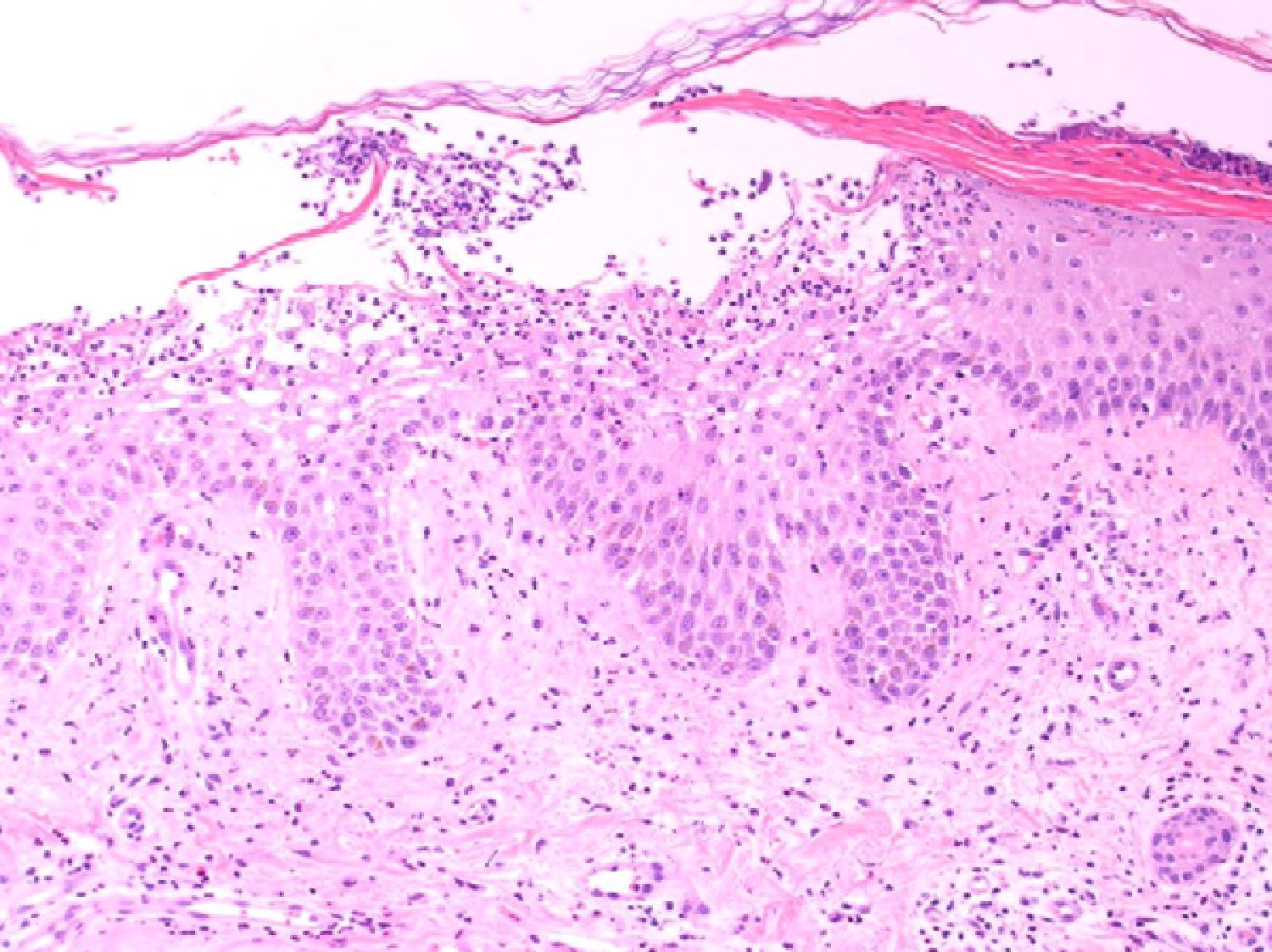
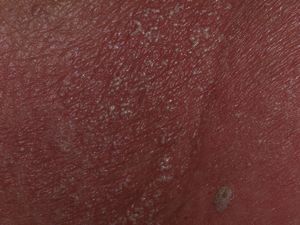
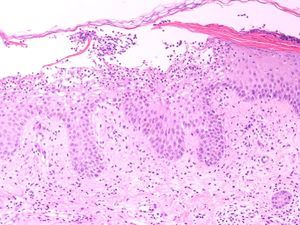
Our patient was an 83-year-old woman with a history of systemic hypertension, diabetes mellitus, and allergy to sulfamides. She was admitted to our hospital for a 1-week history of fever, malaise, and skin rash. The skin lesions had developed 48hours after starting treatment with nystatin mouthwashes for oral candidiasis; this had been the only change to her usual treatment. On admission the patient was febrile (38.3°C). She presented erythroderma and the skin was covered by numerous pustules of 1 to 3mm in diameter; the pustules were not centered on follicles and were more evident on the trunk and roots of the limbs (Fig. 1). The mucosas were not affected and the Nikolsky sign was negative. Blood tests revealed a white cell count of 18 400/μL with neutrophilia (88.9%) but without eosinophilia, and elevation of the acute-phase reactants (erythrocyte sedimentation rate, 63mm; C-reactive protein, 127mg/dL). Blood cultures, the cultures of 2 pustules, and viral serology for rubella virus, cytomegalovirus, Epstein-Barr virus, parvovirus B19, enterovirus, varicella-zoster virus, and human immunodeficiency virus were negative. Biopsy of 1 of the pustules showed a spongiform dermatitis with the formation of subcorneal pustules and a dermal infiltrate rich in polymorphonuclear cells and eosinophils (Fig. 2); the infiltrate was negative to staining with periodic acid-Schiff. The nystatin mouthwashes were discontinued and the patient was administered a short course of oral corticosteroids. She was discharged after 13 days with a good general state of health and presenting only erythema and minimal residual desquamation.
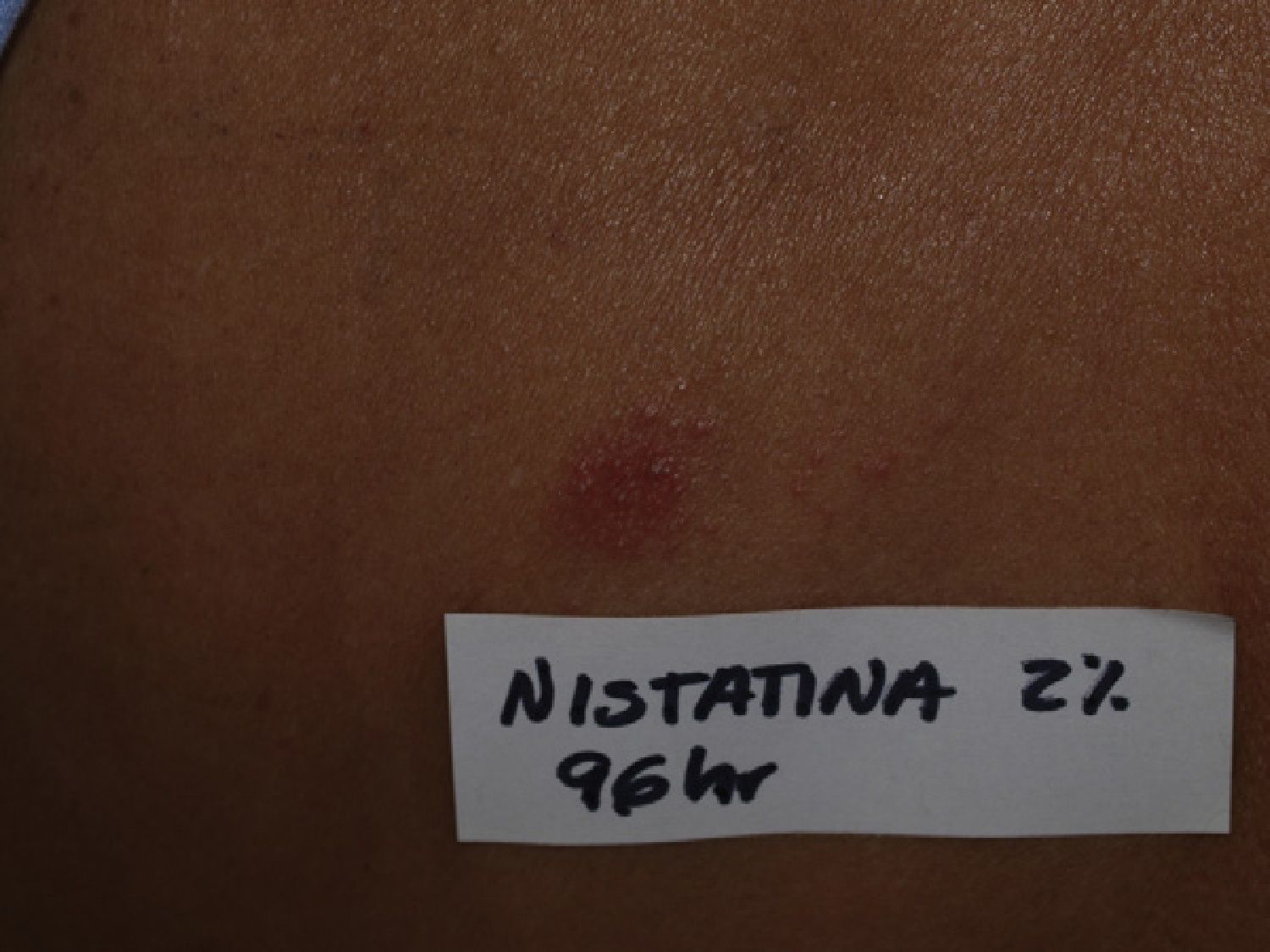
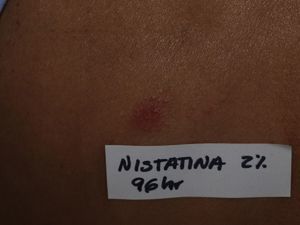
Six months later, patch testing was performed under normal conditions with the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) plus nystatin, 2%, in petroleum jelly (AllergEAZE, Brial Allergen GmbH). Positive results were obtained only with the nystatin patch at 48 and 96hours (Fig. 3); the subsequent study with nystatin, 2%, in petroleum jelly in 11 controls was negative. The reaction caused by the patch in our patient produced the same clinical and histological changes as had been observed initially with the toxic dermatitis, confirming the diagnosis of nystatin-induced AGEP.
Nystatin is a fungistatic drug of the polyenic group, isolated from Streptomyces noursei. It is widely used throughout the world for its efficacy against Candida and for its good tolerance; the main indication for treatment with nystatin as a mouthwash is oropharyngeal candidiasis. Toxic dermatitis due to this drug is rare as it undergoes minimal intestinal absorption after ingestion and is therefore very unlikely to cause systemic reactions. In our patient the clinical presentation (including the temporal relationship with the administration of the drug and clinical course of the lesions) and the histopathological findings were characteristic of AGEP according to current criteria.2
In a review of the literature we found 5 cases of nystatin-induced AGEP.3–6 They were all clinically identical to our case and were also confirmed using various tests, including patch tests and intradermal tests. Our case is the first to be reported in Spain. Other forms of toxic dermatitis caused by oral nystatin, namely generalized dermatitis,7 maculopapular eruption,8 generalized eruption with angioedema,9 and generalized maculopapular rash,10 have been described in case reports. In all the cases, patch testing performed at different concentrations (always >10%) yielded positive results. In our patient we showed that patch testing with a 2% concentration of nystatin is sufficient to make the diagnosis. In general, it is known that the sensitivity of patch testing in the study of AGEP is around 50% (80% for some antibiotics), which is higher than that found in other types of toxic dermatitis, including Stevens-Johnson syndrome.2
Collation of the evidence confirms the participation of drug-specific T lymphocytes in the pathophysiological mechanisms of AGEP, which is a specific form of delayed hypersensitivity.
In conclusion, AGEP should be included in the differential diagnosis of pustular skin eruptions in patients on treatment with oral nystatin, despite its minimal gastrointestinal absorption. Patch testing has high diagnostic value in drug-induced AGEP and other drug-induced forms of toxic dermatitis. In our patient we observed positive results with the drug diluted to lower concentrations than those previously reported in the literature.
Please cite this article as: Ocerin-Guerra I, et al. Pustulosis exantemática aguda generalizada inducida por nistatina. Actas Dermosifiliogr. 2012;103:927–8.