Advanced basal cell carcinomas (BCCs) are thought to account for approximately 1 to 10% of all BCCs, with metastatic tumors accounting for 0.0028 to 0.5%.1 Patients with locally advanced BCC (laBCC) not amenable to surgery or radiotherapy with curative intent are eligible for treatment with targeted agents with palliative intent, such as vismodegib. Treatment discontinuation rates, however, lie around 90%; 33% of discontinuations are due to disease progression and 25% to adverse effects.2–4 One option in such cases is external beam radiotherapy at a median dose of 55Gy (range, 47–85Gy), which has an associated effectiveness of 70%. Response rates in tumors larger than 30mm, however, are just 55%, suggesting that treatment is less effective in larger tumors.5,6 Cytoreductive treatment prior to radiotherapy in patients with laBCC might thus be desirable. Options include vismodegib, surgery, or other destructive strategies such as cryosurgery and curettage-electrodessication.
Recurrence rates of up to 37% have been observed within 6 months of vismodegib discontinuation in complete responders.3,4,7 Consolidation radiotherapy could be considered not only in these patients but also in those who experience disease progression after a partial response.
We describe the cases of 4 patients treated at the National Cancer Institute in Colombia who experienced a complete or partial response or disease progression during treatment with vismodegib. They subsequently achieved complete remission after cytoreductive targeted therapy, combined or not with local destructive methods, and maintained this response after consolidation radiotherapy (Table 1, Figs. 1 and 2).
Characteristics of Patients with Locally Advanced Basal Cell Carcinoma (laBCC) Treated with Multimodal Combination Therapy and Clinical Responses.
| Patient | Age, y/sex | Tumor location/greatest diameter | Treatment before vismodegib | Vismodegib cycles, no. | Response to vismodegib | Adverse effects and grade | Cytoreductive treatments | Radiotherapy type and dose | Response after radiotherapy/follow-up |
|---|---|---|---|---|---|---|---|---|---|
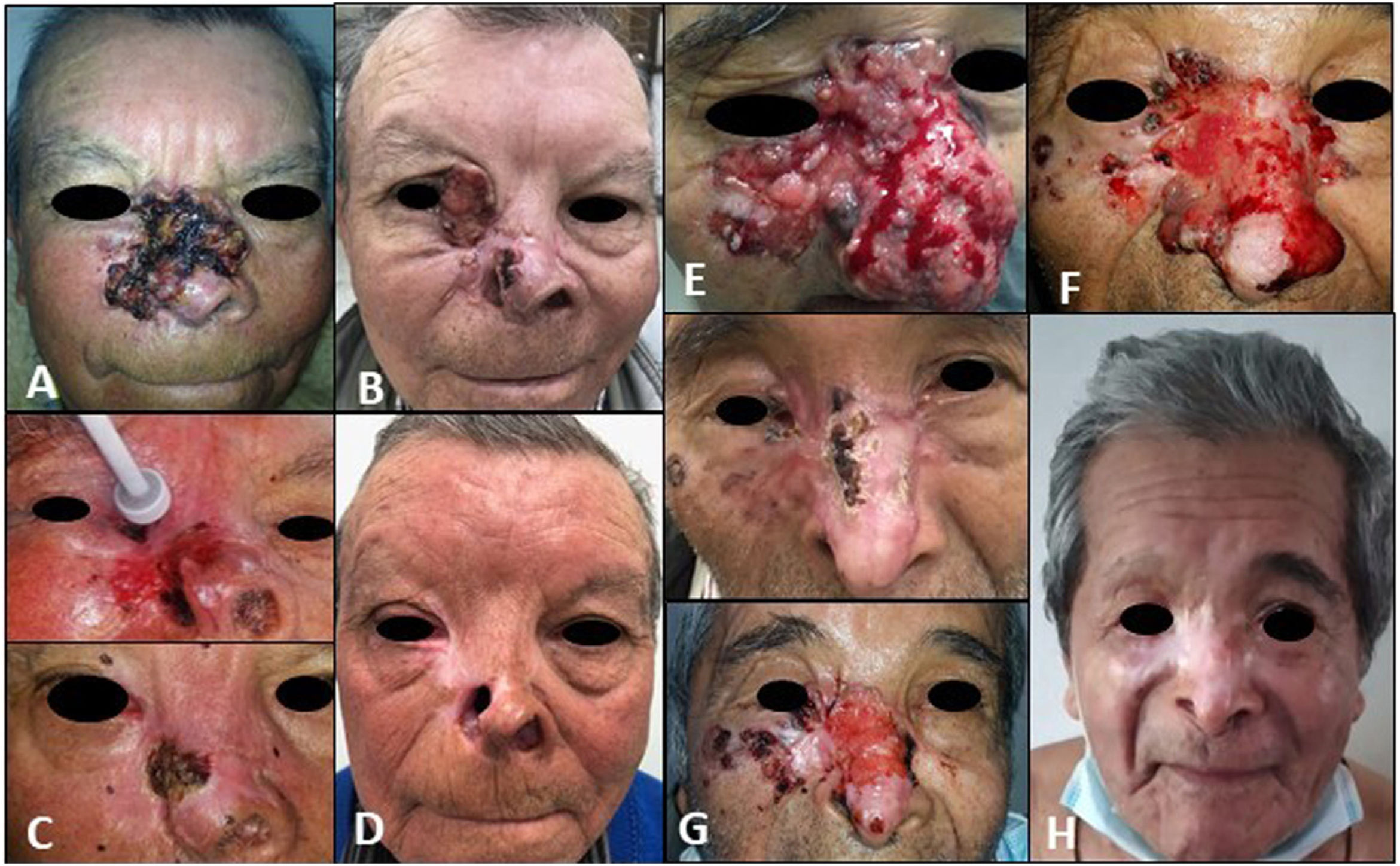
| 1(Fig. 1 A–D) | 69/M | Centrofacial laBCC extending to right orbit/55mm | None | 8 | Progression due to secondary resistance | Grade 2 muscle spasms, grade 1 dysgeusia | Cryosurgery, curettage-electrodessication, topical imiquimod 5%, high intralesional interferon | External beam radiotherapy (66Gy in 33 fractions) after vismodegib discontinuation | Complete clinical and radiologic response/no recurrences during 36 mo of follow-up postradiotherapy (Fig. D) |
| 2Fig. 1 E–H) | 82/M | Centrofacial laBCC extending to right orbit/90mm | None | 37 | Initial partial response followed by disease stabilization (drug discontinued due to toxicity) | Grade 3 muscle spasms, grade 1 dysgeusia | Curettage-electrodessication, cryosurgery, topical imiquimod 5% | External beam radiotherapy (55Gy in 22 fractions) with concurrent vismodegib | Complete clinical response with histologic and radiologic evidence/sustained during 33 mo of follow-up after radiotherapy and 15 mo of follow-up after vismodegib discontinuation (Fig. H) |
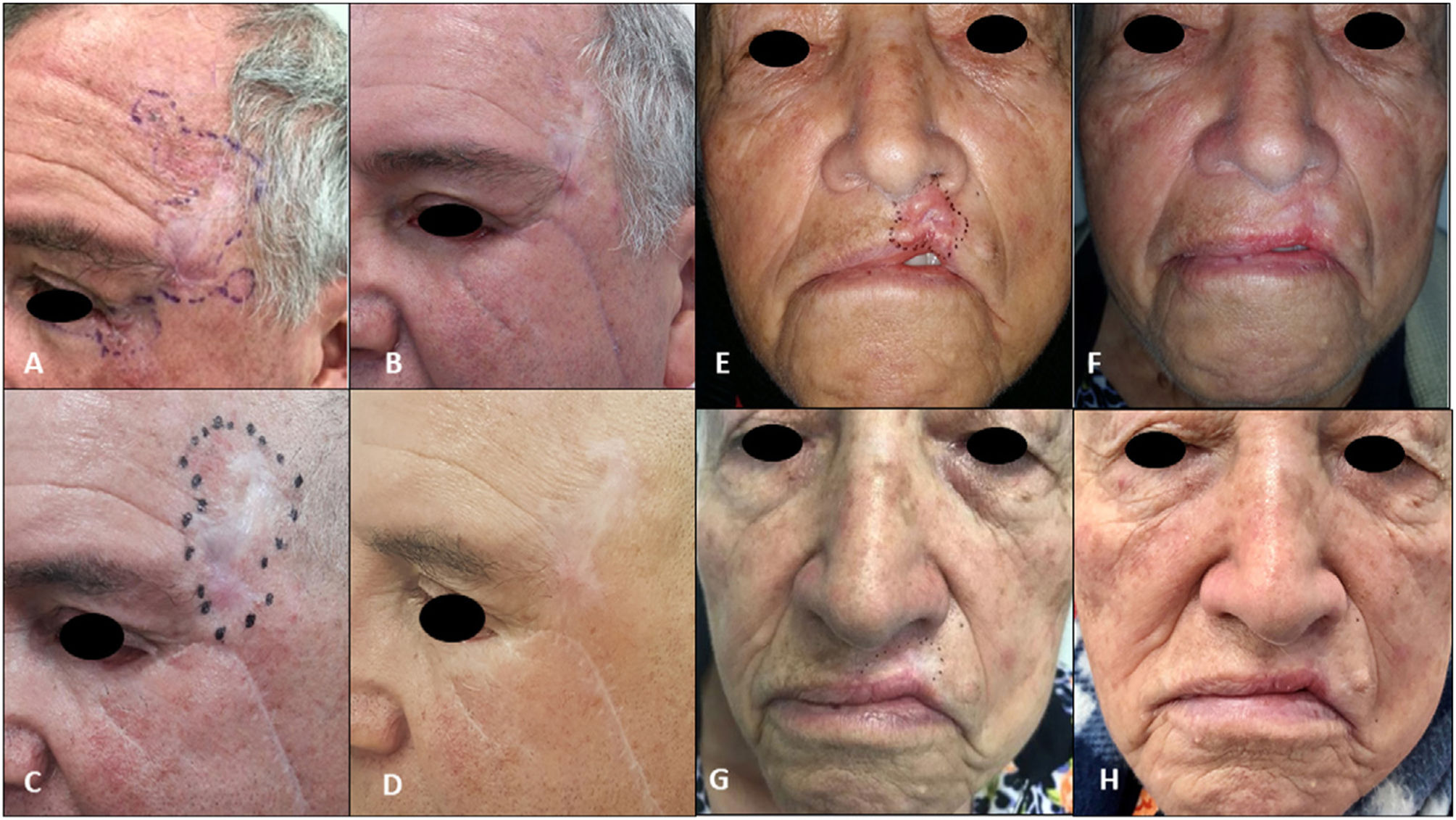
| 3Fig. 2A–D) | 62/M | laBCC in left temporal region extending to outer canthus and lower eyelid of the left eye/50mm | Laser ablation 10 y earlier, local resection plus Mustardé flap with histologically confirmed positive margins 2 mo earlier | 8 | Complete (drug discontinued due to toxicity) | Grade 3 muscle spasms | None | External beam radiotherapy (55Gy in 20 fractions) after vismodegib discontinuation | Complete clinical and radiologic response/sustained during 30 mo of follow-up postradiotherapy (Fig. D) |
| 4Fig. 2E–H) | 82/F | laBCC in skin of upper lip skin/25mm | None | 8 | Complete (drug discontinued due to toxicity) | Grade 3 muscle spasms, grade 1 dysgeusia | None | External beam radiotherapy (55Gy in 22 fractions) after vismodegib discontinuation | Complete clinical and radiologic response/sustained during 34 mo of follow-up postradiotherapy (Fig. H) |
A, Tumor before initiation of vismodegib. B, Partial response in the region of the nasal dorsum and cheek, but progression to the inner canthus of the right eye. C, Tumor cytoreduction with local destructive methods. D, Complete response sustained for 36 months after radiotherapy. E, Tumor before initiation of targeted therapy with vismodegib. F, Increased tumor volume on the wings of the nose and inner canthus after 13 cycles. G, Results after curettage-electrodessication in larger tumor areas and cryosurgery on the wings of the nose. H, Complete remission sustained for 15 months after discontinuation of vismodegib and 33 months after radiotherapy.
A, Tumor before local resection by ocular oncology department at the level of the canthus and eyelid. B, Residual tumor with biopsy-proven positive margins in the left temporal and eyelid region before initiation of vismodegib. C, Complete clinical response after 8 cycles of vismodegib and initiation of external beam radiotherapy. D, Complete remission sustained after 30 months of follow-up. E, Tumor before treatment with vismodegib. F, Complete response after 3 cycles. G, Complete response after 8 cycles and prior to radiotherapy. H, Complete remission after radiotherapy and 34 months of follow-up.
Patient #1 developed secondary resistance to vismodegib and was treated with a combination of cryosurgery and curettage-electrodessication followed by consolidation radiotherapy at a total dose of 66Gy. He achieved a complete clinical and radiologic response and showed no signs of recurrence during 36 months of follow-up. In patient #2, targeted therapy with vismodegib led to stabilization of tumor size with occasional grade 3 muscle spasms. The patient was also treated with concurrent cytoreductive cryosurgery and curettage-electrocoagulation followed by concurrent vismodegib and radiotherapy at a total dose of 55Gy. He achieved a complete clinical and radiologic response and showed no signs of recurrence during follow-up (33 months after radiotherapy and 15 months after discontinuation of vismodegib).
Induction therapy with vismodegib achieved a complete response in patients #3 and #4, but the drug was discontinued due to grade 3 muscle spasms. The patients were treated with sequential schedules of consolidation radiotherapy at a total dose of 55Gy. They both achieved complete remission, with no recurrences observed during 30 and 34 months of follow-up, respectively.
Several series performed in different settings and with follow-up times of up to 15 months have reported complete responses to combination therapy with radiotherapy and vismodegib in patients with laBCC. One of the modalities comprised concurrent vismodegib and radiotherapy followed by oral vismodegib. The combination did not result in an exacerbation of adverse effects. Another modality was a combination trimodal regimen consisting of induction vismodegib followed by radiotherapy and local surgical resection. Induction therapy with vismodegib followed by radiotherapy has also been described.8–10
Yom Sue and colleagues started a phase 2 clinical trial (NCT01835626) in May 2013 to demonstrate the efficacy of a combination approach consisting of induction vismodegib for 12 weeks followed by radiotherapy administered for 5 days over a period of 7 weeks. The results, however, are pending publication.
The cases described in the present series illustrate the viability of a multimodal approach combining induction vismodegib, with or without local cytoreductive strategies, and consolidation radiotherapy. Such an approach may be an excellent option for patients with laBCC and might even achieve lasting complete remission. Studies with greater methodological validity, however, are needed to clarify our findings and draw more solid conclusions on the feasibility of this and expanded indications.
FundingNo funding was received for this study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.