Morphea is an inflammatory, fibrosing skin disorder. When it occurs in childhood, it is also known as juvenile localized scleroderma. It is more common in girls and typically appears around the age of 5 to 7 years. According to a recent classification system, morphea is divided into 5 types: circumscribed (plaque), linear, generalized, pansclerotic, and mixed. Approximately 40% of patients present extracutaneous manifestations. Childhood morphea is treated with phototherapy, oral or topical calcitriol, topical tacrolimus 0.1%, methotrexate, topical or systemic corticosteroids, mycophenolate mofetil, bosentán, and topical imiquimod 5%. A variety of measuring tools are used to monitor response to treatment. Few prognostic studies have been conducted, but findings to date suggest that the disease tends to run a chronic or intermittent-recurrent course and frequently causes sequelae.
La morfea es una enfermedad de la piel que se manifiesta en forma de inflamación y fibrosis. En niños y jóvenes, también se conoce como esclerodermia juvenil localizada. En edad infantil, afecta con mayor frecuencia al sexo femenino y la edad de comienzo se ha establecido en torno a los 5-7 años. Una clasificación reciente divide la morfea en: circunscrita (en placas), lineal, generalizada, panesclerótica y mixta. Alrededor de un 40% de los pacientes presentan manifestaciones extracutáneas.
Los tratamientos empleados en morfea infantil son: fototerapia, calcitriol oral, calcipotriol tópico, tacrolimus 0,1% tópico, metotrexato, glucocorticoides tópicos y sistémicos, mofetil micofenolato, bosentán e imiquimod 5% tópico. Diversas medidas de resultado pueden ayudar a monitorizar el tratamiento. Los estudios pronósticos son escasos, pero apuntan hacia una enfermedad con tendencia a un curso crónico o intermitente-recurrente y una frecuencia considerable de secuelas.
Morphea is a fibrosing inflammatory skin disorder. When it occurs in children and adolescents, the condition is also known as juvenile localized scleroderma to distinguish it from the systemic form of the disease (called juvenile systemic sclerosis or juvenile systemic scleroderma). When the term morphea is used there is no way, equivalent to juvenile localized scleroderma, of specifically denoting the disease in childhood or adolescence. In this review, we will use the term morphea in childhood to refer to the condition in the pediatric population.
Epidemiology, Etiology, and PathogenesisThere are very few studies on the incidence and prevalence of morphea. In a study of the incidence of childhood scleroderma in the United Kingdom and Ireland between 2005 and 2007, researchers found an annual incidence rate of 3.4 cases per million children for morphea of all types and 2.5 per million for linear morphea (LM).1 The authors of a US study on the epidemiology of morphea in Olmstead County (Minnesota) between 1960 and 1993, found an annual age- and sex-adjusted incidence rate per 100,000 population of 2.7 cases for morphea and 0.5 for linear morphea.2 Patients who were under 18 at diagnosis accounted for 34% of the patients with morphea and 69% of those with LM.
Childhood-onset morphea affects girls more than boys, with a female-to-male ratio of 2-3:1.3,4 Age of onset has been established at between 5 and 7 years.5,6 Mean age at diagnosis ranges from 7 to 13 years.3,4,6 In a series of 750 children with morphea, the mean interval between onset and diagnosis was 1.6 years (median 11 y, range 0-16.7 y).3 In a series of 52 patients with LM, this interval was 1.8 years (range, 15 d to 8 y).6 Other studies have identified a delay of 9 to 11 years.4,5,7
A family history of rheumatic and autoimmune diseases is reported in between 12.1%3 and 24.3%8 of cases. This proportion falls to between 6%3 and 8.8%8 in first-degree relatives. The diseases most frequently associated with morphea are rheumatoid arthritis, scleroderma, systemic lupus erythematosus and thyroiditis.3,8 The skin diseases most often associated with morphea are psoriasis (16.3%),vitiligo (2.3%), and lichen sclerosus et atrophicus (0.8%).3
Environmental factors, that is events occurring close to disease onset, have been reported in between 13.2%8 and 13.3%3 of patients. The most common were mechanical events (8.9%), including traumas and insect bites.3
The etiology and pathogenesis of morphea are poorly understood. The condition appears to be caused by interactions between inflammatory, fibrotic, and vascular processes. One hypothesis that would explain how fibrosis is triggered in morphea is the transformation of CD34+ fibrocytes into CD34- myofibroblasts and an increase in XIIIa1+ dermal dendrocytes.9 Some authors have reported the almost complete disappearance of CD34+ dermal dendritic cells and an increase in factor XIIIa1+ dermal dendrocytes in fibrosed lesions in patients with active morphea.10
Clinical Presentation, Classification, and Extracutaneous ManifestationsTraditionally, morphea has been classified into 5 subtypes: plaque or circumscribed, linear, generalized, bullous, and deep (including subcutaneous morphea).11 The Pediatric Rheumatology European Society (PReS) has proposed a more exclusive and precise classification (Table 1).12,13 They propose replacing the term plaque morphea (PLM) with the term circumscribed morphea. This form can be further divided into superficial and deep subtypes (Table 1). In the present review, we have used the term plaque morphea because it is the one used in the studies we cite, most of which were carried out before the PReS classification was proposed.
Clinical Forms of Morphea According to the Pediatric Rheumatology European Society (PReS) Classification.
| Subtype (frequency)a | Description |
|---|---|
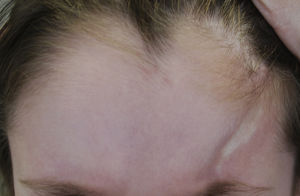
| Linear morphea (LM) (most common form in childhood: 51%8-65%3) | One or more linear indurations affecting a variety of sites: LM of the trunk or limbs: typically unilateral. Involves the dermis, subcutaneous cell tissue and, sometimes, muscle or underlying bone. LM on the face and scalp: • Morphea en coup de sabre: linear induration on the forehead and/or scalp, usually unilateral and paramedian, sometimes involving muscle and underlying bone. Can extend to areas below the forehead (Fig. 1) • Parry Romberg Syndrome or progressive hemifacial atrophy: characterized by loss of tissue on one side of the face below the forehead. Loss may affect the dermis, subcutaneous tissue, facial muscles, and even the parotid gland and underlying bone. In PRS, the epidermis is often spared. |
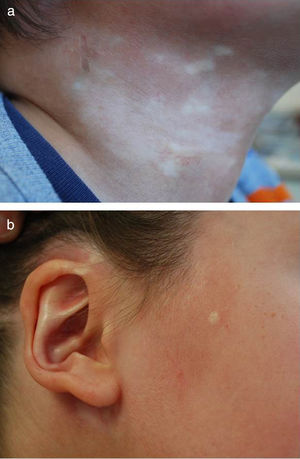
| Circumscribed morphea (plaque morphea) (26%3-37%8 of cases of morphea in children) | Oval or round circumscribed areas (plaques) of induration surrounded by a violaceous halo and mainly affecting the trunk (Figs. 1A and 1B There are two subtypes: • Superficial plaque morphea in which the lesions are localized to the dermis, although it may occasionally affect the uppermost layer of subcutaneous tissue. • Deep plaque morphea, in which the lesions extend from the dermis into the subcutaneous tissue and may involve the fascia or underlying muscle. |
| Mixed morphea (found in up to 15% of patients3) | Combination of 2 of more of the above subtypes. The proposed nomenclature would include, in brackets, the component subtypes ordered according to the extent of involvement in each case (with the more predominant component first). For example, mixed morphea (linear-circumscribed)13 (Figs. 1 and 2) |
| Generalized morphea (7%3-8%8 of cases in children) | Presence of four or more plaques larger than 3cm in diameter, which become confluent and involve at least 2 out of 7 anatomic sites (head-neck, right upper extremity, left upper extremity, posterior trunk, right lower extremity, and left lower extremity).12,13 Some authors set a threshold for involvement of at least 30% of body surface.8 |
| Pansclerotic Morphea (0,27%3) | Circumferential full-thickness involvement of skin, subcutaneous tissue, muscle, and bone affecting the trunk, limbs, face, or scalp, with sparing of the palms and soles. This subtype does not affect internal organs. Possibility of complications, including the appearance of chronic ulcers and squamous cell carcinomas. |
The classification proposed by PReS also includes the term mixed morphea (MM), a variant that appears to be more common than previously believed. This decision indicates a shift towards viewing morphea as a term encompassing a spectrum of clinical variants characterized by inflammation and fibrosis occurring at different depths (Figures 1 and 2). Another factor supporting this view is the overlap between morphea en coup de sabre (ECDS) and Parry-Romberg syndrome (PRS) or progressive hemifacial atrophy, a condition that occurs in between 24% and 48% of patients.4,14,15 The PReS classification does not, however, recognize certain other subtypes, such as the bullous, deep, and subcutaneous forms of morphea.11 Traditionally, deep morphea (DM) is characterized by thickening of the skin, subcutaneous tissue, and fascia. It is a rare form, appearing in around 4% of patients with morphea.8 Subcutaneous morphea is defined by incipient involvement of subcutaneous tissue. Bullous morphea is characterized by the appearance of edema and tense dermal or subepidermal bullae secondary to lymphatic obstruction caused by fibrosis, which can also lead to edema in the limbs.16 The PReS classification proposes the inclusion of these forms as new morphea subtypes (depending on the clinical presentation)12 and the exclusion of eosinophilic fasciitis,11 lichen sclerosus et atrophicus, and atrophoderma of Pasini and Pierini as forms of morphea at the extreme end of the spectrum.17
Five-year-old boy with mixed morphea (circumscribed-linear ECDS) as defined by the PReS classification. The circumscribed component is clearly visible in the plaques situated on a) the right side of the neck, and b) the right preauricular and parotic regions, including involvement of the pinna.
Extracutaneous manifestations are observed in up to 40% of patients with morphea (Table 2).8
Extracutaneous Manifestations of Morphea.
| Extracutaneous Manifestations of Morphea |
|---|
| Musculoskeletal Involvement |
| • Arthralgias (16.9%)8 are the most common extracutaneous manifestation. They can occur in almost any of the morphea subtypes and are very common in patients with LM affecting the limbs and, less frequently, in patients with GM or PLM. |
| • Contractures and asymmetrical or abnormal growth8 are found in LM affecting the limbs. |
| • Scoliosis and thoracic asymmetry.8 |
| Neurological Involvement |
| • Headache is the most frequent neurological manifestation, particularly in patients with ECDS.8 |
| • Epileptic seizures appear in in 8% to 21% of patients with ECDS and PRS.3,4,7 |
| • Abnormal electroencephalogram findings are seen in patients with ECDS and PRS, taking the form of frontal, temporal, frontotemporal, and generalized abnormalities. |
| • Morphological alterations on head CT: bony deformities in the form of thinning of the affected overlying region4,18 |
| • Abnormal brain MRI findings: focal cerebral atrophy, gray-white matter blurring, and signal abnormalities on T2-weighted images in the area below the affected skin4 |
| • Others: stroke and peripheral neuropathies8 |
| Note: Patients with no neurological symptoms may also demonstrate anomalies on brain imaging, but less frequently.7 |
| Gastrointestinal Involvement |
| • Cases of reflux and dysphagia have been reported.8 |
| Pulmonary Involvement |
| • Dyspnea secondary to restrictive lung disease has been reported in patients with DM, GM and LM.8 |
| Concomitant Autoimmune Diseases |
| • Concomitant autoimmune diseases have been reported in 9.6% of patients with morphea, including vitiligo, alopecia areata, psoriasis, juvenile rheumatoid arthritis, juvenile dermatomyositis, and Crohn disease.8 |
| Ophthalmological Manifestations6 |
| • Ocular involvement has been reported in 3.2% of patients, occurring mainly in ECDS (66.7%). |
| • Abnormalities of the eyelids or adnexa (41.7%) |
| • Abnormalities of the anterior segment (uveitis, episcleritis) (29.2%) |
| • Less common abnormalities include paralytic strabismus (1 patient), pseudopapilledema (1 patient) and refractive errors (2 patients) |
| Dental Abnormalities |
| • Maxillary and mandibular hypoplasia have been reported in PRS.7 |
Abbreviations: CT, computerized tomography; DM, deep morphea; ECDS, morphea en coup de sabre; GM, generalized morphea; LM, linear morphea; MRI, magnetic resonance imaging; PLM, plaque morphea; PRS, Parry-Romberg syndrome.
No correlation has been observed between abnormal laboratory test results and the activity, course, or prognosis of the disease in patients with morphea.3,8 Elevation of acute phase reactants is more common in the deeper forms of morphea, in LM, and during the inflammatory phase of the disease. An elevated white blood cell count is found in 37.5% of patients and an increased erythrocyte sedimentation rate in 25%.3 Peripheral blood eosinophilia is seen in up to 62.6% of patients with DM, falling to between 12% and 18.5% in patients with other clinical forms of the disease.3,8 Creatinine phosphokinase can be elevated in the deeper forms and this anomaly is associated with muscle pain and weakness.3 Some patients present abnormalities in immune electrophoresis of serum proteins associated with elevation of diverse immunoglobulins.3 Antinuclear antibodies have been found in up to 42.3%3 of patients; they are observed more frequently in LM, generalized morphea (GM), and the deeper forms of the disease.3,8 Rheumatoid factor is detected in 16% of children with morphea.3
TreatmentThere are numerous options for the treatment of morphea, including phototherapy, topical corticosteroids, topical calcipotriol, oral calcitriol, topical tacrolimus, systemic corticosteroids, methotrexate, mycophenolate mofetil, intralesional gamma interferon, ciclosporin A, D-penicillamine, imiquimod, bosentan, infliximab, etanercept, adalimumab, hydroxychloroquine, and photopheresis.18,19 In many cases there is insufficient evidence to support the use of these treatments in this setting, even in the case of drugs as widely used as topical corticosteroids.19 The variation observed in the treatments used is remarkable and tends to be related to the specialty (dermatology or rheumatology) of the prescribing physician.19 To date, there has been only 1 systematic review of studies on the treatment of patients (children and adults) with morphea.20 In this review we only mention drugs that have been studied in children and adolescents (Table 3).
Therapeutic Options for Morphea in Childhood.
| Treatment | Level of Evidence (SIGN) |
|---|---|
| Low-dose UV-A1 | 1- |
| Medium-dose UV-A1 | 1- |
| Narrowband UV-B | 1- |
| PUVA bath | 3 |
| Oral PUVA | 3 |
| Oral calcitriol | 3 |
| Topical calcipotriol | 1- |
| Topical tacrolimus 0.1% | 3 |
| Methotrexate/systemic corticosteroids | 1+ |
| Mycophenolate mofetil | 3 |
| Bosentan | 3 |
| Topical imiquimod 5% | 3 |
| Surgical Intervention | 3 |
Levels of evidence. 1++: high quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias. 1+: well-conducted meta-analyses, systematic reviews of RCTs, and RCTs with a low risk of bias. 1-: meta-analyses, systematic reviews of RCTs, and RCTs with a high risk of bias. 2++: systematic reviews of high quality case-control studies, or case-control studies and high quality cohort studies with a very low risk of confounding, bias, or chance and a high probability that the relationship is causal. 2+: well-conducted case control or cohort studies with a low risk of confounding, bias, or chance and a moderate probability that the relationship is causal. 2-: case control and cohort studies with a high risk of confounding, bias, or chance and a significant risk that the relationship may not be causal. 3: non-analytical studies (case reports, case series). 4: expert opinion, consensus documents.
Abbreviations: PUVA, psoralen-ultraviolet A; SIGN, Scottish Intercollegiate Guidelines Network.
The findings of numerous studies support the use of phototherapy in morphea; most of the evidence comes from uncontrolled, open-label studies and case series. While few children were enrolled in the studies, the efficacy of low-dose UV-A1, medium-dose UV-A1, narrowband UV-B, and topical psoralen-UV-A (PUVA) have all been assessed in this age group. We found only one study that enrolled only children.21
The only randomized controlled trial (RCT) in patients with morphea compared the efficacy of low-dose UV-A1, medium-dose UV-A1, and narrowband UV-B phototherapy.22 The authors of that study did not blind the assessment of the primary outcome (the Modified Skin Score). The study enrolled 66 patients with PLM, LM, ECDS, DM, and GM aged between 5 and 73 years. All three phototherapy regimens achieved statistically significant reductions in the Modified Skin Score and improved histological and ultrasound findings. Medium-dose UV-A1 was more effective than narrowband UV-B, but no differences were found between low- and medium-dose UV-A1, or between low-dose UV-A1 and narrowband UV-B.
An open-label prospective study assessed the efficacy of low-dose UV-A1 (20J/cm2) in 19 pediatric patients with PLM, LM, PRS, and DM.21 In addition, all the patients applied calcipotriol ointment (0.005%) twice daily. A statistically significant reduction of 67.1% was observed in the Modified Skin Score following treatment. Two children reported mild skin irritation, which was attributed to calcipotriol.
The authors of a controlled intrapatient study that compared the efficacy of medium-dose and low-dose UV-A1 in patients with PLM found significant differences between the 2 regimens in the ultrasound assessment.23
PUVA-bath (8-methoxy-psoralen) photochemotherapy was used in an uncontrolled open-label prospective study.24 The study included 17 patients between 9 and 72 years of age with PLM, LM, and GM. The authors reported improvement in 13 of the patients both on a clinical scale and in terms of ultrasound and histologic indications.
Finally, isolated cases of pansclerotic morphea in childhood treated with oral PUVA25,26 and UV-A phototherapy have been reported.27
Vitamin D DerivativesTopical calcipotriol was used in association with low-dose UV-A1 in the study mentioned above.21
The efficacy of oral calcitriol (1,25-dihydroxycholecalciferol) was assessed in an uncontrolled open-label prospective study of 7 pediatric patients with LM; the lesions improved in 5 of the 7 children according to a clinical scoring system.28 No adverse effects were reported. In a double-blind RCT, oral calcitriol did not demonstrate greater efficacy than placebo in 27 adults.29
Topical ImmunomodulatorsTacrolimus 0.1% has been used with good results in several small studies with adults.30,31 However, a case of bullous morphea in childhood that did not respond to treatment with tacrolimus 0.1% has also been published.16
In an open-label study, 9 children with PLM were treated with imiquimod 5% cream for 9 months.32 Imiquimod reduced both the mean score on the visual analog scale (VAS) and skin thickness on high-resolution ultrasound. However, the reduction observed on the DIET score (depigmentation, induration, erythema and telangiectasia) was not statistically significant. One patient experienced ulceration that required temporary discontinuation of treatment.32
MethotrexateMethotrexate (MTX) is often used to treat the different forms of morphea in childhood. The usual regimen is a dose of 10 to 15mg/m2/w, administered orally or subcutaneously33–35 (0.3-0.6mg/kg).36 This regimen is often complemented by folic acid and, during the early weeks of treatment, an induction phase of systemic corticosteroid therapy, generally in the form of intravenous boluses of methylprednisolone at a dose of 30mg/kg/d (pulsed intravenous corticosteroid combined with methotrexate therapy [PCMT]).6,33,35,36 Oral prednisone may be added to this regimen.33 The combination of MTX and corticosteroid therapy appears to be more effective than the use of either drug alone.6 Treatment duration varies, and patients may continue on this regimen for several years.6,34,37 Data on 34 cases of children with morphea treated with PCMT for between 1 and 3 years was analyzed in a retrospective study.33 The regimen stopped disease progression in 94% of the patients and 24 patients (71%) had no signs of active disease at the end of the follow-up period (0.2-7 years). In 16 (47%) patients, therapy was discontinued when the disease was considered to be inactive (a mean of 20 ± 12 months). Of these, 7 (44%) experienced a relapse following withdrawal of treatment.
A double-blind RCT published in 2011 compared the efficacy of oral MTX 15 mg/m2 and placebo.34 Pediatric patients were randomized to receive MTX or placebo once weekly, for 12 months or until treatment failure. Both groups also received oral prednisone 1 mg/kg/d for the first 3 months. A target lesion was selected for clinical assessment with thermography and a computerized scoring system based on a skin score rate (SSR) evaluation. Response to treatment was defined by 3 criteria: 1) a decrease in temperature of at least 10% over baseline; 2) SSR of at least 1; and 3) the absence of new lesions. Relapse was defined as a failure to meet any 1 of these criteria. The study enrolled 70 patients aged between 6 and 17 years with various morphea subtypes (44 with LM, 18 with GM, and 8 with MM). Statistically significant differences between the 2 groups were found in thermography and SSR, but not in the appearance of new lesions. Relapse occurred in 46% of the patients who completed the 12-month treatment period (32.6% in the MTX group and 70.8% in the placebo group; P < .005). The side effects reported were mild and in no case led to discontinuation of treatment. In an open-label extension of that study using the same response criteria, 65 patients were treated with MTX 15 mg/m2 for 12 months, followed by a tapered regimen of MTX.37 On follow-up, 35 of the 65 patients maintained clinical remission for a mean of 25.6 months (median 24, range 6-48). Of the 65 patients, 10 did not respond to treatment, 8 relapsed before completing 12 months of MTX, 5 relapsed when the regimen was tapered, and 7 were lost to follow-up.
Mycophenolate MofetilIn spite of the lack of evidence to support its efficacy, mycophenolate mofetil is frequently used by rheumatologists to treat morphea.19 In a series of 10 patients with morphea in childhood, patients were switched to mycophenolate mofetil when their condition had not responded satisfactorily to PCMT after at least 4 months of treatment.38 Subjective clinical improvement and thermographic changes were reported. None of the patients developed new lesions during treatment. Of the 10 children, induration diminished in 9 and a reduction in erythema was observed in 7.
Miscellany. Other treatments reported in the literature include bosentan in pansclerotic morphea39 and surgical correction of ECDS using techniques such as simple excision,40 dermal fat grafting41 and deep filling with a sodium hydroxyapatite biomaterial.42
Monitoring Treatment (Outcome Measures)As morphea is a rare disease, standardized and validated outcome scales are useful for assessing and comparing the efficacy of different treatments.43 The results of a survey of pediatric dermatologists and rheumatologists in the United Kingdom revealed that they were not all using the tool considered most appropriate for monitoring these patients and that the tools used varied greatly depending on the physician's specialty.19
- •
Clinical Scales
Numerous studies of morphea treatments have used clinical scales, but these instruments are rarely used in clinical practice.19 The more recent scales have been validated. Two of the most used scales are the Modified Skin Score (MSS)22,35,44,45 and the Modified Rodnan Skin Score (MRSS)19,46 (Table 4).
Clinical Scales for the Assessment of Morphea.
| Authors | Scale | Description | Advantages and Disadvantages | Validation | |
|---|---|---|---|---|---|
| Seyger 199745 | MSS (Modified Skin Score) | The body is divided into 7 anatomical regions. The degree of skin thickening and the percentage of body surface affected in each region are scored from 0 to 3 (total score 0-42). | May not differentiate active and inactive lesions because only skin thickness is measured. Measures the affected area in each region, but does not relate it to the total body surface area (making the scale of little use in children). Low correlation with durometer values because the MSS includes area of involvement as well as pliability | MSS vs durometer Assessment of: Skin thickening (0-3) % of affected area: 0 (0%), 1 (<33%), 2 (33%-67%), 3 (> 67%) | MSS: Interobserver variability 2.2% Intraobserver variability 0.0% Durometer Interobserver variability 0.5% Intraobserver variability 0. Correlation between total MSS and durometer: 0.5 |
| Porta 201446 | MRSS (Modified Rodnan Skin Score) | Assesses skin thickness in 17 surface anatomical areas of the body using a 0-3 scale. | May not distinguish between active and inactive lesions because it only measures skin thickness. Overestimates the involvement of hands and fingers (in systemic sclerosis) Measures the affected area but does not relate it to the total body surface area (making it of little use in children). Good agreement with ultrasound. | mRSS vs Ultrasound (18MHz) Assessment of skin thickening | Agreement: ICC 0.8889; KW 0.783 |
| Zulian 200747 | CSS (Computerized Skin Score) | Assesses total area affected and area of both active and inactive lesions. | Takes into account the affected body surface area (useful in children). Dedicated software package. Not very useful for lesions with poorly defined borders or very extensive lesions. It is sometimes difficult to differentiate between active and inactive lesions. | Assessment of the area of activity and the area of induration | Interobserver agreement 95%. Intraobserver agreement 91%-93%. The ICC for the indurated area among experts was 0.97 and among residents 0.91-0.94. |
| Arkachaisri 200848 | LoSSI (Localized Scleroderma Skin Severity Index) | Scores the following from 0 to 3 in 14 anatomical areas: extension, erythema, thickening and new lesions. | Useful for assessing morphea during the initial, inflammatory phase | LoSSI PhysGA-A PtGA | LoSSI interobserver agreement: 95.56% (KW 0.83). Intraobserver agreement: 93.65%, 88.89% (KW 0.83, 0.66) Correlationa between LoSSI and PhysGA 0.44 Correlation between PhysGA/PtGA: 0.27 (95% CI, 0.0-0.64). |
| Arkachaisri 200949 | mLoSSI (modified LoSSI) | Similar to the LoSSI but does not take into account the surface area affected | Useful for evaluating morphea during the early, inflammatory stage The surface area is excluded to increase the degree of interobserver agreement. | mLoSSI PhysGA-A PtGA CDLQI | mLoSSI: Interobserver agreement: ICC 0.8 Intraobserver agreement: rs = 0.77 PhysGA: interobserver: ICC 0.90 PtGA: interobserver: ICC 0.63 |
| Arkachaisri 201050 | LoSDI (Localized Scleroderma Damage Index) | Presence of dermal atrophy, subcutaneous atrophy, and dyspigmentation. Score of 0 to 3 in 18 anatomical areas | Useful for assessing the damage caused by morphea | LoSDI PhysGA-Damage PtGA | LoSDI: Interobserver agreement: ICC 0.99 Intraobserver agreement: rs = 0.96. PhysGA-D: Interobserver agreement: KW 0.90 Intraobserver agreement: rs = 0.89 Correlationa LoSDI with PGA-D: 0.58 Correlation PhysGA-D and PtGA: 0.42 and 0.46 |
| Pope 201132 | DIET (Dyspigmentation, Induration, Erythema and Telangiectasia) | Presence of dyspigmentation, induration, erythema, and telangiectasias | May not detect active lesions that have become residual. Good correlation with high-resolution ultrasound (13-MHz) and LoSSI | DIET VAS | DIET: intraobserver agreement: ICC 0.75 VAS: interobserver agreement: ICC 0.59 VAS: agreement between parents and researchers: ICC 0.5 |
Abbreviations: CDLQI, Children's Dermatology Life Quality Index; ICC, intraclass correlation coefficient; KW, kappa-weighted coefficient; PhysGA, physician global assessment of disease activity; PtGA, patient global assessment of disease activity; rs, correlation coefficient.
A clinical scale that uses a computerized methodology has been developed: the Computerized Skin Score (CSS).47 In this technique, an adhesive transparent film is applied over the lesion and the borders of the active and inactive areas of morphea are outlined used two different colors. The resulting image is scanned and processed by a computer program that can differentiate between the two colors and calculate the total area of the lesions and of the active and inactive zones, as well as the total body surface affected. To date, the CSS has only been used in 1 RCT, which assessed the efficacy of treatment with MTX.34,37
The Localized Scleroderma Skin Severity Index (LoSSI) and the Physician Global Assessment of Disease Activity (PGA-A) are used to assess morphea during the inflammatory phase.48 The modified LoSSI (mLoSSI) does not take into account the affected surface, thereby resulting in greater inter- and intraobserver agreement.49 More recently, we have seen the development of tools designed to determine residual damage: the Localized Scleroderma Damage Index (LoSDI) and the Physician Global Assessment of Disease Damage (PGA-D). By combining the scores of the mLoSSI, the PGA-D, and the LoSDI, the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) can evaluate both disease activity and residual damage in patients with morphea.50 To improve the use of such measures, an atlas has been developed containing photographs of lesions representing different levels of activity and damage.51 A study that evaluated the LoSCAT in a pediatric population has demonstrated the validity of the mLoSSI and PGA-A as measures of disease activity in morphea.52
The VAS methodology is based on assessments by both the patient and the researcher.22,49,50 Finally, the DIET scale53,54 has demonstrated good correlation with VAS, LoSSI, and high-resolution ultrasound (13-MHz).32,55
- •
Quality-of-Life-Scales
Very few authors have studied quality of life in children with morphea. The authors of a cross-sectional cohort study found that morphea had a moderate impact on the patients’ quality of life. The instruments used in that study were the Child Health Assessment Questionnaire, the Child Dermatology Life Quality Index, the Child Quality-of-Life Questionnaire, and the Child Health Questionnaire.56
- •
Laboratory Testing
While laboratory test results in patients with morphea may reveal a number of changes, these do not appear to correlate with the activity, course, or prognosis of the disease.3,8 Several proinflammatory molecules are detected in active morphea and correlate with the number of affected areas and active lesions; however, they are of no practical use in patient monitoring.43
- •
Ultrasound Imaging
A number of authors have used high-resolution ultrasound as one of their outcome measures.21–24,57 The dermis affected by morphea lesions can be studied using a 20 MHz probe. Wavelengths of 8 to 15 MHz may be required to study subcutaneous cell tissue, fascia and muscle.58 Using linear probes of 7 to 15 MHz, some studies have achieved a sensitivity and specificity for ultrasound diagnosis of morphea of 100% and 98.8%, respectively.59,60 Increased subcutaneous tissue echogenicity and increased blood flow (using Doppler) were the most accurate sonographic sighs of activity (100% sensitivity and specificity for each variable, with respect to histological examination).60 It is important to note that ultrasound determinations can vary depending on differences in the equipment used and the sonographer's interpretation. Protocols have been developed to minimize these differences, including the Ultrasound Disease Activity Score.58
- •
Thermography
Thermography has been used as an outcome measure in several studies.33,34,38 A lesion is defined as active in thermography when an area is identified as having a temperature difference of at least 0.5°C with respect to the surrounding and contralateral skin.61 Several studies attributed thermography a sensitivity of between 80% and 92% and a specificity of between 68% and 77% in the detection of active lesions.62,63 Specificity appears to be limited by the presence of older, sclerosed lesions, which may appear as “hot”, especially in areas where the subcutaneous cell tissue is atrophied and in regions, such as the face and scalp, where subcutaneous tissue is scarce.62,63 One study raised the sensitivity to 100% and the specificity to 80% when thermography was associated with clinical assessment and used in regions where there was no significant subcutaneous cell tissue atrophy.64
- •
Magnetic Resonance Imaging
Magnetic resonance imaging can detect dermal thickening during the active phases of the disease. Changes in the dermis, fascia and muscle appear as a hypointense signal on T1-weighted images without contrast enhancement. If there is bone involvement, the signal is detected in T2-weighted images and on T1-weighted images with contrast enhancement. MRI changes have been observed in patients with DM, LM, GM and pansclerotic morphea with and without suspicion of musculoskeletal involvement.65,66 However, as MRI is an expensive imaging technique offering only poor resolution in the dermis, it seems reasonable to limit its use to patients with severe forms of morphea.
- •
Cutometer and Durometer Measurements
The cutometer is a device used to measure skin elasticity and pliability. Although cutometers have been used to assess outcomes in several studies,67,68 the use of these devices to evaluate morphea has not yet been validated.
The durometer is a device that measures skin hardness. In pediatric patients with morphea, durometer readings have demonstrated good inter- and intraobserver agreement, even in observers with different levels of training.69 Furthermore, they help to differentiate active and inactive lesions and can detect changes in the lesions over time. However, studies have found only a moderate correlation between durometer findings and the MRSS, LoSSI, and Modified Skin Score0,45,69 and a weak correlation with the mLoSDI scale.69
PrognosisAlthough prognosis in patients with morphea has been little studied and long-term studies are particularly scarce, the available evidence suggests that the disease tends to run a chronic or intermittent-recurrent course and frequently causes sequelae. In the short and medium term, systemic treatment with MTX and/or corticosteroid therapy appears to stop the progression of the disease and/or improve lesions.6,33,34,37 However, the condition recurs in between 28% and 46% of patients within 20 months of cessation of treatment.33,34,70 Activity persists in between 28% and 89% of patients in the long term.6,71 In a survey of patients with LM conducted after follow-up of up to 20 years, all but one of the respondents reported having residual damage.6 This included cosmetic sequelae in 66% and functional limitations in 38%. In total, 51% of those surveyed considered that the treatment they had received had been effective. Finally, individuals who develop morphea in early childhood may have decreased quality of life and be more likely to develop autoimmune conditions in later life.71
ConclusionsThe term morphea encompasses a broad spectrum of clinical forms which have considerable potential for causing long-term sequelae, making early diagnosis and appropriate treatment very important.
Although little used in routine clinical practice, instruments for determining morphea activity are a useful aid when making therapeutic decisions. The existing clinical scales appear to reflect the state of the disease objectively and correlate one with another; however, their use requires more time than is usually available in routine clinical practice. High-resolution ultrasound has high sensitivity and high specificity for morphea, but nonetheless requires time, specific equipment, and appropriate training.
The treatments for children supported by the highest quality of evidence (based on epidemiological studies) are phototherapy and the PCMT regimen. The form of phototherapy used in most of the studies in the literature was low-dose UV-A1, an option not readily available in Spain. However, 1 study found no differences between low-dose UV-A1 and narrowband UV-B. However, long-term outcomes in patients treated with phototherapy have not been documented.
In summary, PCMT has been shown to be effective in the control of morphea in the short and medium term. However, since morphea is a chronic disease characterized by intermittent outbreaks and periods of remission, new treatment strategies and long-term follow-up studies are needed.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Aranegui B, Jiménez-Reyes J. Morfea en la infancia: actualización. Actas Dermosifiliogr. 2018;109:312–322.