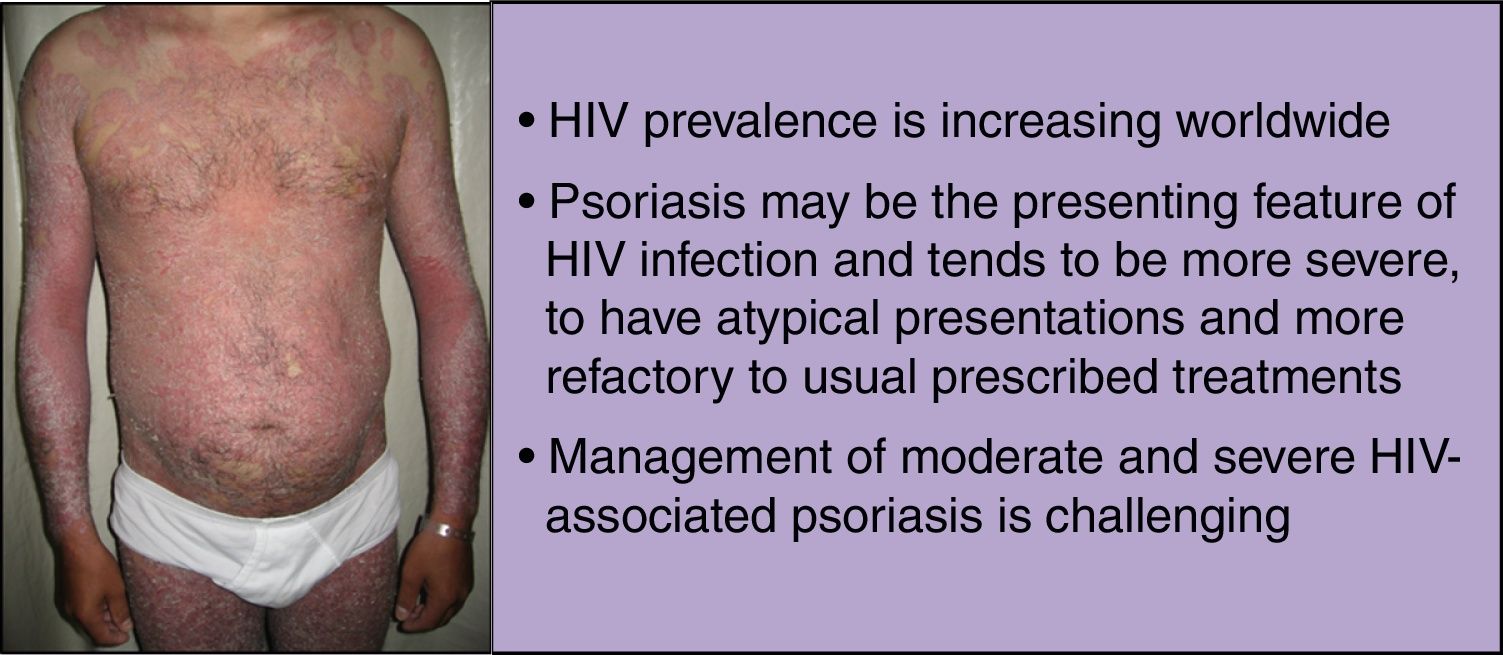
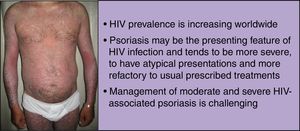
Human immunodeficiency virus (HIV) prevalence is increasing worldwide as people on antiretroviral therapy are living longer. These patients are often susceptible to debilitating inflammatory disorders that are frequently refractory to standard treatment. Psoriasis is a systemic inflammatory disorder, associated with both physical and psychological burden, and can be the presenting feature of HIV infection. In this population, psoriasis tends to be more severe, to have atypical presentations and higher failure rates with the usual prescribed treatments.
Management of moderate and severe HIV-associated psoriasis is challenging. Systemic conventional and biologic agents may be considered, but patients should be carefully followed up for potential adverse events, like opportunist infections, and regular monitoring of CD4 counts and HIV viral loads.
La prevalencia del virus de la inmunodeficiencia humana (VIH) va en aumento en todo el mundo ya que las personas en tratamiento antirretroviral cada vez viven más años. Estos pacientes suelen ser propensos a trastornos inflamatorios debilitantes que suelen ser refractarios al tratamiento estándar. La psoriasis es un trastorno inflamatorio asociado a una carga física y psicológica, y puede ser una característica de presentación de infección por VIH. En esta población de pacientes, la psoriasis suele ser más grave, tener presentaciones atípicas e índices más altos de fracaso a los tratamientos que suelen prescribirse.
El manejo de la psoriasis asociada al VIH de carácter moderado y grave es todo un reto. Pueden considerarse agentes convencionales y biológicos, pero debe someterse a los pacientes a un meticuloso seguimiento para descartar posibles episodios adversos tales como infecciones oportunistas, así como una monitorización habitual de los recuentos de CD4 y cargas virales de VIH.
HIV prevalence is increasing worldwide as patients on antiretroviral therapy (ART) are living longer. In 2012 an estimated 35.3 million people were living with HIV.1
Dermatological disorders, such as psoriasis, can be the presenting feature of HIV infection. Moreover, in the HIV-infected population, psoriasis tends to be more severe (with severe disease correlating with higher immunosuppression),2,3 to have atypical presentations and higher failure rates with the usual prescribed treatments.4 Although mild psoriasis is usually manageable, moderate to severe disease is more difficult to treat, and side effects from immunosuppressive drugs, such as, opportunistic infections, are the main concern.
This review discusses the pathogenesis, clinical features and management of HIV-associated psoriasis.
PsoriasisPsoriasis is a systemic inflammatory disorder,5 associated with both physical and psychological burden.6 Five types of psoriasis have been described: plaque-type psoriasis, guttate psoriasis, inverse psoriasis, pustular psoriasis (either localized or generalized pustular psoriasis), and erythrodermic psoriasis.5 Concomitant psoriatic arthritis occurs in up to 30% of patients.7 Psoriasis is also associated with several important comorbidities, including crohn's disease, cancer, depression, non-alcoholic fatty liver disease, metabolic syndrome (or its components), and cardiovascular disease (CVD).8,9 These associations might be due to similarities in the genetic basis and due to systemic inflammation mainly seen in severe psoriasis.10
Involvement of both innate and adaptive immune system in psoriasis is widely accepted.11,12 Various immune cells, including dendritic cells, macrophages, several subsets of T-cells, neutrophils, mast cells, keratinocytes and others, produce a wide range of pro-inflammatory mediators, such as, IFN-α, TNF-α, IL-1β, IL-6, IL-20, IL-23, chemokines and anti-microbial peptides which initiate, amplify and sustain the psoriatic inflammatory cascade.13–31
HIVThere are two types of HIV: HIV-1 and HIV-2. HIV-1 has marked genetic diversity as a consequence of the error-prone function of reverse transcriptase, resulting in a high mutation rate. HIV-2 is largely confined to West Africa, less transmissible and although it causes a similar illness, immunodeficiency progresses more slowly.32
The hallmark of HIV infection is the progressive depletion of CD4 T cells due to reduced production and increased destruction. CD4 T cells are eliminated by direct infection, and bystander effects of syncytia formation, immune activation, proliferation, and senescence. In early infection, a reduction in circulating CD4 T cells is followed by recovery to near normal concentrations, which slowly decrease by about 50-100 cells/μL per year.33 HIV infection is also characterized by a marked increase in immune activation, which includes both the adaptive and innate immune systems.34 The drivers for immune activation include the direct effects of HIV as a ligand for the Toll-like receptor (TLR7 and TLR 8) expressed on plasmacytoid dendritic cells, leading to production of interferon-α35; microbial translocation, with lipopolysaccharide as a potent activator of TLR4 leading to the production of proinflammatory cytokines such as IL-6 and TNF-α36; co-infection with viruses that induce profound expansion of activated T cells;37and a reduced ratio of T-helper-17 and regulatory T cells, especially in the gastrointestinal tract.38 Evidence of residual inflammation or increased immune activation exists, even in patients with HIV with adequate CD4 T cell restoration on ART. figures 1-2
Aging with HIV is often linked to non-infectious comorbidities, including CVD, hypertension, type 2 diabetes mellitus, chronic kidney disease, osteopenia/osteoporosis, and non-AIDS cancers. These heterogeneous comorbidities share age and presumably HIV-infection as independent risk factors, and tend to aggregate into complex multi-morbidity patterns.39,40 The pathophysiology of HIV-associated CVD includes an intricate interplay of inflammation, direct effects of HIV proteins, immune dysfunction, drug effects, malnutrition, and other factors. Inflammation and dyslipidemia drive the pathophysiology of coronary artery disease (CAD) in patients infected with HIV. In particular, persistent HIV viral replication,41 microbial translocations,42 and coinfections (eg, cytomegalovirus) contribute to a proinflammatory milieu that accelerates atherosclerosis.37 The development of ART transformed HIV from an illness with a fatal outcome to a chronic manageable disease. Standard regimens combine two nucleoside reverse transcriptase inhibitors with a non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or integrase inhibitor. After initiation of ART, the plasma viral load decreases to concentrations below the lower limit of detection of available commercial assays in most people within 3 months. By contrast, the recovery of CD4 T cells in treated individuals is variable.43 However, prolonged ART use predisposes to dyslipidemia, hyperglycemia, and lipodystrophy, among other side effects.44 Protease inhibitors (PIs), particularly older generation PIs, are commonly implicated in ART-associated dyslipidemia, and are associated with greater risk of acute myocardial infarction.45,46 They are postulated to increase CVD risk by inducing dyslipidemia and reactive oxygen species production, with resultant mitochondrial dysfunction, fatty infiltration in liver and muscle, and insulin resistance.47 In high income countries, these effects, compounded with the cardiac effects of aging and inflammation, contribute to an increased risk of numerous CVDs.44 On the other hand, in regions where ART initiation is often delayed and access is limited, the predominant manifestations are still related to opportunistic, infection-related myopericardial disease.48
Psoriasis and HIVHIV-associated psoriasis has a similar or greater prevalence than that of seronegative psoriasis in the general population.49 A recent nationwide population-based cohort conducted in Taiwan showed that HIV may increase the risk for developing incident psoriasis. This study included 102,070 patients (20,294 HIV-patients and 81,776 matched controls) and showed that after adjusting for age, sex, and comorbidities, HIV infection was an independent risk factor for incident psoriasis (adjusted hazard ratio, 1.80; 95% confidence interval: 1.38 to 2.36).50
Interestingly, it has been shown that genetic variants that contribute to anti-viral immunity may predispose to the development of psoriasis, as psoriasis patients are significantly more likely to have gene variants protective against HIV-1 disease. It was also found that the compound genotype KIR3DS1 plus HLA-B Bw4-80I, which respectively encode a natural killer cell activating receptor and its putative ligand, significantly increase psoriasis susceptibility and is associated with delay of progression to AIDS.51 Moreover, multiple genes associated with psoriasis (RNF114, TNFAIP3,TNIP1, MDA5 and RIG-1) have been shown to regulate antiviral signaling.52–55 However, this anti-viral effect is probably compartmentalized to the skin, and psoriasis has a systemic antiviral effect. In fact, a recent retrospective cohort study demonstrated that concurrent psoriasis and HIV infection is associated with a statistically higher but clinically non-significant serum viral load set point than having HIV without psoriasis.56 Also some psoriasis-associated variants in MHC Class I and Class III regions have been found to influence host control of HIV-1 infection. HIV-1 control is a rare immunologic phenotype (1% of HIV-1 infected individuals) in which patients who are infected with the HIV-1 virus spontaneously maintain low viral loads in the absence of anti-retroviral therapy. Two psoriasis-associated MHC Class I single nucleotide polymorphisms (rs9264942 and rs3021366) were associated with both HIV-1 controller status and viral load, while another Class III MHC variant (rs9368699) was associated with viral load. Other genetic variants outside the MHC (SOX5, TLR9, SDC4, PROX1, IL12B, TLR4, MBL-2, TYK2, IFIH1) were also shown to possibly influence, however it and warrants further research.57
Psoriasis has been observed in all stages of HIV infection and in some patients, it may be the initial presentation of the infection. In patients with pre-existing psoriasis, exacerbation of the disease is common when HIV infection occurs.49 Exacerbations of psoriasis by staphylococcal and streptococcal infection are also more common in patients with HIV than in those without, and heavy colonization usually prolongs the disease course.58
In HIV context, T-cell imbalance, may be responsible in part for the development of worsening of psoriasis, due to the depletion of CD4 suppressor T cells, resulting in unchecked proinflammatory pathways.59 Furthermore, HIV RNA transcripts have been identified in the skin of patients with HIV-associated psoriasis and within CD4 Factor XIIIa+ dermal dendritic cells, which suggests a direct role of HIV and is compatible with worsening disease with the higher viral loads associated with progressive immunodeficiency.60 HIV might also directly trigger psoriasis as a costimulatory factor through antigenic presentation or as a source of superantigens.59 In HIV-associated psoriasis HIV Negative Regulatory Factor (nef) protein has superantigen properties.61 Moreover, infections by virus and bacteria, which are common in HIV, might contribute to epitope spreading.60
Psoriasis has also been reported as a manifestation of HIV-related immune reconstitution inflammatory syndrome (IRIS). IRIS is an exacerbation or emergence of a previously unknown disease resulting from rapid restoration of pathogen-specific immune responses to pre-existing antigens combined with immune dysregulation, which happens shortly after initiation of ART. IRIS happens more commonly when ART is started in patients with low CD4 counts or soon after starting treatment for opportunistic infections. Strategies to reduce IRIS include initiation of ART at high CD4 counts, delayed initiation in patients with an infection, and screening for and prevention of opportunistic infections.62 There is emerging evidence that one of the immunopathogenic mechanisms of IRIS involves the rapid and dysregulated shift from the Th2 predominant state of advanced HIV infection to the Th1 and Th17 dominant state of immune recovery.63
As discussed above, HIV-infection and psoriasis are conditions in which CVD are an important group of comorbid diseases. As such, screening, monitoring and treatment of cardiometabolic risk factors and CVD is important in psoriasis patients infected with HIV, since these patients are more prone to develop such diseases.
Clinical FeaturesIncidence rates of psoriasis in HIV patients are similar or higher to those reported in the general population.4 However, misleading and unusual clinical presentations, severe disease, and frequent exacerbations are characteristic.58 Although all clinical subtypes of psoriasis may occur in patients with HIV-associated psoriasis, guttate, inverse, and erythrodermic occur with higher frequencies.2,3,64 Often, more than one morphological type of psoriasis may coexist in a single patient, which is a distinguishing hallmark feature of HIV-associated psoriasis.3
As in non-HIV patients, plaque-type psoriasis is the most common form of the disease, and accounts for about 90% of cases. However, in this specific population there is a predilection for scalp lesions, palmoplantar and flexural involvement, as well as a trend towards severe immunodeficiency with CD4 counts below 200 cells/mm3.4,65 On the other hand, different clinical variants can develop over the course of the disease. Often there is no family history of psoriasis.60 In cases of sudden exacerbation of psoriasis (serious, extensive and inflammatory forms) of psoriasis, HIV infection should be suspected.49
Sebopsoriasis is more common in patients with HIV. The clinical features represent an overlap between seborrheic dermatitis and psoriasis.66 Sebopsoriasis can be triggered by yeasts, and the effectiveness of systemic or topical antifungals is helpful in distinguishing this variant from classic psoriasis.67
Rupioid psoriasis is one of the rarer variants of psoriasis, and has also been reported as a manifestation of HIV-associated psoriasis.68 This variant is characterized by limpet-like, cone-shaped, hyperkeratotic nodules and plaques covered with an exudative crust typically presenting on the limbs.60
Psoriatic arthritis is much more destructive and refractory to conventional treatments in the HIV population, and its prevalence is also greatly increased compared with its immunocompetent counterpart.69,70 Reactive arthritis prevalence is also higher in patients with HIV. This disease is a seronegative spondyloarthropathy characterized by a triad of arthritis, urethritis, and conjunctivitis.71 A reactive arthritis-like psoriasis syndrome is a recognized presentation in HIV-associated psoriasis.58 The typical cutaneous presentation of this variant, seen in more than half of patients, is keratoderma blennorrhagica with palmoplantar psoriasiform plaques. The histology is identical to that of pustular psoriasis.60
Diagnosis and differential diagnosisCutaneous diseases are common manifestations of HIV infection and AIDS, and may be indicative of the degree of immune dysfunction. Psoriasis can be the presenting feature of HIV infection and can provide a clinical clue as to the state of the patient's immune system.72 The patient's history should include details of family members with the disease, and potential trigger factors, such as present infections or new medications.73 As in non-HIV patients, psoriasis diagnosis is usually made on clinical findings and skin biopsy is rarely needed.74 Joint involvement should also be inquired, especially in HIV patients. In the presence of the extremely rare rupioid psoriasis, physicians should suspect immunosuppression, such as HIV/AIDS, or other immune dysregulaton.72
The most common differential diagnosis of psoriasis includes tinea infection, seborrheic dermatitis and eczema of several causes. Less common differential diagnosis includes lichen planus, pityriasis rubra pilaris (PRP), pityriasis rosea and cutaneous lymphoma.65 While PRP is a rare disease that can be associated with HIV infection (type VI) and is characterized by follicular keratotic papules or plaques that may coalesce to form erythematous or salmon-colored plaques that can develop scales,75 cutaneous non-Hodgkin's lymphomas are rare in patients with HIV-1 infection and almost all of the cases reported are of T-cell lineage with histopathological features of mycosis fungoides or Sèzary syndrome.76
HIV-related CD8+ cutaneous pseudolymphoma should also be considered in patients with HIV, especially in those who are severely immunocompromised and may resemble psoriasis, as it usually presents as extensive plaques or erythroderma. Histologically they may mimic cutaneous T-cell lymphoma. Highly active antiretroviral therapy (HAART) is considered as the first-line treatment, particularly in deeply immunocompromised patients, but methotrexate has been reported to be effective and safe in moderately immunocompromised patients.77,78
Papulosquamous secondary syphilis might clinically and histologically resemble psoriasis and can be distinguished by other clinical clues of syphilis, and positive syphilis serology. A high index of suspicion is needed since false negative syphilis serology might initially be encountered due to the so-called prozone phenomenon associated with high treponemal loads with HIV.60
Crusted scabies, to which patients with HIV are prone, and is highly contagious if undiagnosed may be excluded using microscopy. The clinical presentation can mimic psoriasis since the lesions present as scaly plaques on the skin, scalp and nails.79
In cases of nail psoriasis, care must be taken to exclude onychomycosis, since the clinical features can be similar and is common in HIV patients.60
Some patients with erythrodermic psoriasis have large scales resembling either lamellar ichthyosis or a chronic erythrodermic drug eruption. Patients infected with HIV are particularly prone to the latter; hence, a careful history and a high index of suspicion are needed. These patients can develop generalized skin failure associated with higher mortality.60
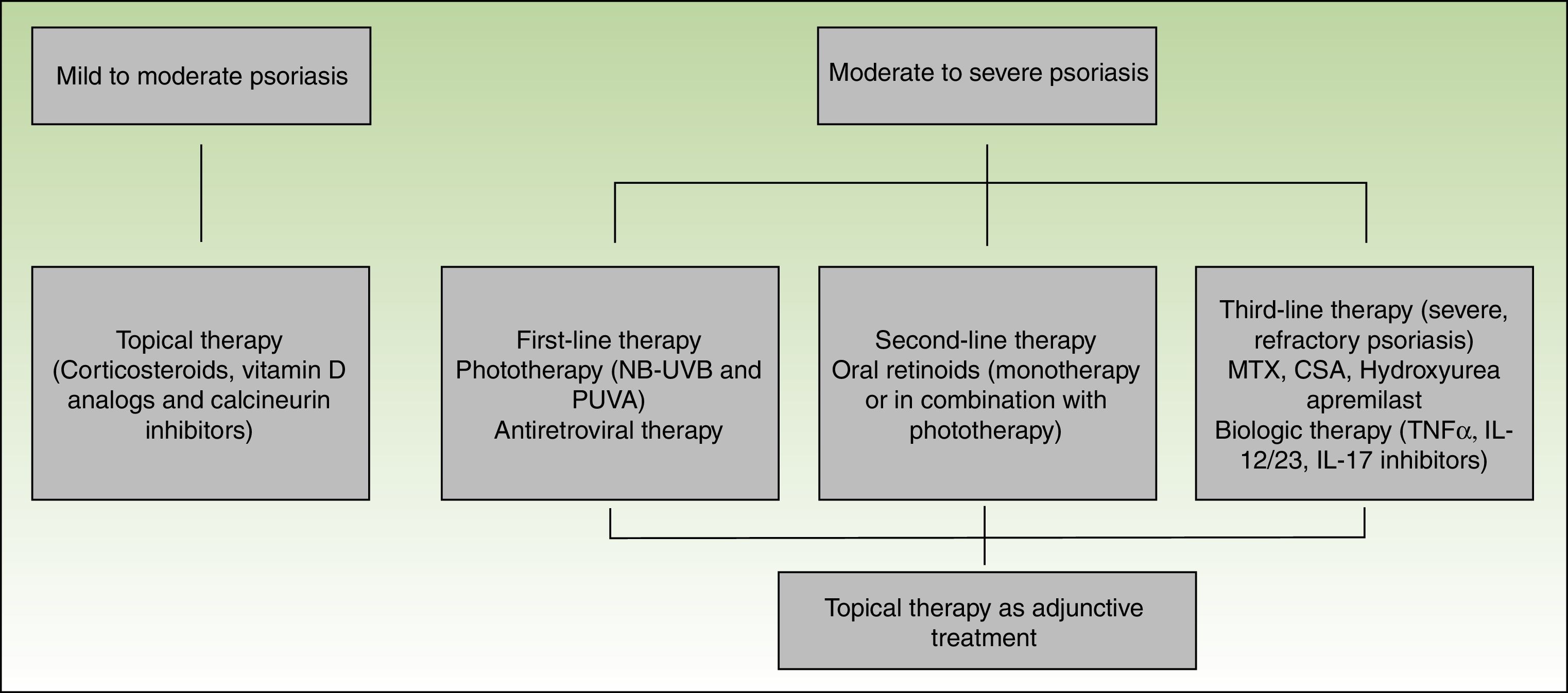
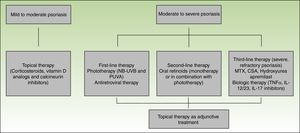
TreatmentThe treatment of psoriasis in HIV-infected patients poses a distinct therapeutic challenge as HIV-associated psoriasis is a T-cell mediated disease in the setting of T-cell depletion. Many of the systemic treatments for psoriasis are immunosuppressive and potentially can lead to severe complications in the setting of HIV infection.3 Moreover, specific guidelines for management and treatment of HIV-infected psoriasis patients are still lacking as most evidence is limited to case reports, and small series of patients, lacking randomized clinical trials.
Topical therapies may be used alone or in combination with other treatment modalities, including phototherapy and systemic agents. These therapies are appropriate for treatment of mild to moderate disease or as an adjunctive treatment for moderate to severe disease.80
Corticosteroids, vitamin D derivatives, or combinations of both are usually sufficient to manage mild disease. Topical calcineurin inhibitors are used for difficult to treat sites, such as the intertriginous areas or the face.65 Side effects from topical therapies in HIV patients are comparable with the non-HIV-infected population, and similar caution should be exercised when using these agents.80
Phototherapy (narrow-band UVB and PUVA) and/or systemic therapy is needed for patients with moderate to severe disease. Both UVB and PUVA have been used in the treatment of HIV-associated psoriasis. As in the non-HIV infected population, phototherapy is clinically effective and provides substantial relief in patients with debilitating disease.80 The carcinogenic potential of PUVA limits its long-term use.65 A potential problem associated with the use of UV light is photosensitivity. Patients with HIV are prone to photosensitivity either directly due to HIV or ART.81 Care must be taken when selecting patients since there is a raised incidence of non-melanoma skin cancer and melanoma in patients receiving more than 250 treatments of PUVA82,83 but overall UVB is thought to be safe.79
In HIV patients, ART should also be considered as a first-line therapy for moderate to severe psoriasis, and in some patients, it can be used as monotherapy.80 Improvement of HIV associated psoriasis after the initiation of ART has been reported in several case reports.84–86
As second-line therapy for moderate to severe psoriasis, systemic oral agents are recommended. Oral retinoids are the most commonly used and represent an effective, non-immunosuppressive alternative. Retinoids are successful as monotherapy or as part of combination regimens, mainly with UV therapy. An additional effect is that lower cumulative doses of UV can be used. Dosing for HIV patients is similar to the non-HIV, although higher doses may be required to achieve optimal results.87,88
In refractory patients immunosuppressants such as methotrexate (MTX), cyclosporine (CSA) and hydroxyurea are an option; however, in the HIV-infected population opportunistic infections are a concern. Although MTX is a key treatment in classic psoriasis and has been used for HIV-associated psoriasis, it is hepatotoxic and can cause leucopenia and opportunistic infections, hence it should be used with caution.60
Cyclosporine exerts its immunomodulatory effect by inhibiting the activation of CD4 T cells via calcineurin inhibition. In published cases of treatment of HIV-associated psoriasis with CSA, patients experienced rapid clearing of psoriasis without the development of opportunistic infections or worsening of immunosuppression.89,90 On the other hand, careful monitoring for nephrotoxicity and hypertension is needed. Cyclosporine is recommended for short intermittent courses of up to 12 weeks. It can also be used when rapid remission is needed in potentially lethal psoriasis variants such as erythroderma or generalized pustular psoriasis.60
Hydroxyurea is a systemic immunosuppressive drug. In addition to its anti-psoriatic effects, it has also been shown to have antiviral effects. This dual effect makes it a drug to consider when treating HIV-associated psoriasis. There are multiple reports that discuss the safety and efficacy of hydroxyurea in psoriasis and HIV separately. A 2011 review suggests that hydroxyurea is generally safe and effective. The main risk involves the hematologic adverse events (anemia, leukopenia, thrombocytopenia, and macrocytosis) which appear to be dose-dependent. Despite this fact, hydroxyurea may be considered as a viable option for patients with generalized psoriasis inadequately responsive to other safer options, whether the patient is HIV-positive or not.91
Apremilast is an oral small-molecule PDE4 inhibitor that works intracellularly blocking the degradation of cAMP, increasing intracellular cAMP levels in PDE4-expressing cells. This inhibition results in the reduced expression of proinflammatory mediators, and an increased expression of anti-inflammatory mediators, providing apremilast an anti-inflammatory rather than immunosuppressive mode of action.92 Apremilast has not been studied in HIV-associated psoriasis, however it has been recently reported the successful and safely use of apremilast in a psoriasis patients with HIV and hepatitis C.93 Due to its mechanism of action and less immunosuppression, apremilast may be a good option for HIV-associated psoriasis.
In the past decade, several biologic agents have been developed and approved for the treatment of psoriasis and psoriatic arthritis. TNF-α inhibitors include etanercept, adalimumab, and infliximab that are approved for the treatment of psoriasis and psoriatic arthritis, and golimumab that has been approved for psoriatic arthritis. Ustekinumab is a p40-IL12/23 inhibitor and is approved for both indications while secukinumab and ixekizumab are IL-17A inhibitors and have been approved for the treatment of psoriasis (secukinumab and ixekizumab) and psoriatic arthritis (secukinumab). Biologics agents are used for long-term treatment because there is no evidence of cumulative toxicity or drug–drug interactions. Furthermore, these agents have a good safety profile with only a small increase in opportunistic infections. These agents are usually used as a second-line treatments, however it is in part due to their high costs.65
The American Academy of Dermatology reviewed 27 published cases of patients with HIV treated with TNF-α inhibitors. Although their review is limited because no randomized controlled trials have been performed with this population, it is proposed that reliable seropositive patients, who are adherent to medication regimens and frequent monitoring and have failed other treatment modalities, should be considered for treatment with TNF-α inhibitors, as anti-TNF-α agents have been used effectively in patients with HIV-associated psoriasis and HIV-associated psoriatic arthritis unresponsive to a combination methotrexate and ciclosporin.94–96 TNF-α is a pivotal cytokine in the pathogenesis of granulomatous inflammation, stimulates HIV transcription in vitro97 and is thought to be involved in the pathogenesis of aphthous ulcers, cachexia, dementia, fatigue and fever in HIV. A concern about the use of this therapy is the predisposition to other infections. If TNF-α inhibitors are to be used, other comorbidities that are often noted in HIV, such as tuberculosis, hepatitis B, and other opportunistic infections, need to be ruled out. Hepatitis C infection, however, is not seen as a contraindication to the use of TNF-α inhibitors because they have been used safely and effectively in patients with hepatitis C infection.60,98 Regarding ustekinumab there are reports of its use in refractory HIV-associated psoriasis, which has been described as safe and efficient, and without side effects or decrease in CD4 counts99,100 while the use and safety of secukinumab and ixekizumab have yet to be established in this population. Recently, it has been reported the use of TNF inhibitors and ustekinumab in 10 Italian patients. The mean duration of therapy was 34.8 months and none of the patients developed adverse effects, including infectious complications, or had to interrupt the treatment. All patients showed overall stable or slightly decreased levels of CD4, which subsequently resolved without changing the antiretroviral therapy.101
ConclusionsThe immunological dysfunction associated with HIV creates an environment that is favorable to develop psoriasis. The main postulated mechanisms are T-cell imbalance and HIV being a source of superantigens, and a costimulatory factor through antigenic presentation.
Treatment of HIV-associated psoriasis can be challenging, and should be based on disease severity and the risks and benefits of each treatment should also be considered carefully. Randomized clinical trial are still lacking as well as specific guideline for this group of patients. Recommended first line treatments include topical therapy, phototherapy, ART and secondarily oral retinoids. For refractory disease, cautious treatment with systemic immunosuppressants should be considered. Biologic agents may prove to be a good option in these cases. Regardless of therapy, HIV patients should be followed up carefully for potential adverse events, with regular monitoring of CD4 counts and HIV viral loads.
Please cite this article as: Queirós N, Torres T. Psoriasis asociada a VIH. https://doi.org/10.1016/j.ad.2017.09.014