Methotrexate (MTX) is the most frequently used conventional systemic drug in the treatment of psoriasis. Despite over 50years of experience in this setting, certain aspects of the use of this drug in clinical practice are still little standardized and poorly understood. For this reason, a group of 15 experts took part in a consensus development conference to achieve consensus on a series of recommendations on the use of MTX in psoriasis. The guidelines, which were developed on the basis of a systematic review of the literature, were validated by 2 rounds of voting and categorized by level of evidence and grade of recommendation. Before MTX can be used to treat moderate to severe psoriasis, the patient must be evaluated to assess the suitability of the treatment, including consideration of vaccination status and screening for tuberculosis and pregnancy. The recommended starting dose for a patient with no risk factors is 10 to 20mg/wk, the therapeutic dose for most patients is 15mg/wk, and the maximum dose is 20mg/wk. Most patients who respond to treatment will show improvement within 8weeks. Parenteral administration of MTX is desirable when there is a risk of erroroneous dosing, nonadherence, gastrointestinal intolerance, or inadequate response to the therapeutic dose taken orally. Noninvasive methods are preferred for monitoring hepatotoxicity. MTX is a good treatment option for patients with a history of cancer, but is not recommended in patients with chronic hepatitisB infection or individuals who are seropositive for human immunodeficiency virus.
Metotrexato (MTX) es el fármaco sistémico convencional más empleado en el tratamiento de la psoriasis. A pesar de que la experiencia en su uso se remonta a más de 50años, todavía existen aspectos en el manejo clínico poco estandarizados o conocidos. Bajo esta premisa, un grupo de 15 expertos participó en una conferencia de consenso en la que, a partir de una revisión sistemática y 2 rondas de validación previas, se validaron recomendaciones categorizadas por nivel de evidencia y grado de recomendación sobre el uso de MTX en la psoriasis.
La elección de MTX en el tratamiento de la psoriasis moderada grave requiere la evaluación previa de la idoneidad del fármaco, incluyendo estado de vacunación, cribado de tuberculosis y gestación. La dosis recomendada de inicio es de 10–20mg/semana si el paciente no presenta factores de riesgo, con una dosis terapéutica de 15mg/semana para la mayoría de pacientes y máxima de 20mg/semana. La mayor parte de pacientes que respondan al tratamiento mostrarán mejoría antes de las 8 semanas. Es preferible la administración parenteral de MTX cuando exista riesgo de error en la pauta de administración, incumplimiento, intolerancia gastrointestinal o respuesta insuficiente a dosis plenas por vía oral. Los métodos no invasivos son preferibles para la monitorización de la hepatotoxicidad. El tratamiento con MTX representa una buena opción en pacientes con antecedentes oncológicos, mientras que no se recomienda en pacientes portadores crónicos de virus de la hepatitisB o seropositivos para el virus de la inmunodeficiencia humana (VIH+).
Methotrexate (MTX) is the most frequently used conventional systemic drug in the treatment of psoriasis, for which—in moderate to severe cases—it is still considered a first-line treatment, both in monotherapy and in combination with other drugs. The evidence supporting the use of MTX in moderate to severe psoriasis is based on retrospective studies and more than 50 years of clinical experience rather than on the results of clinical trials or controlled prospective studies.1,2 Although a good level of evidence can be found in some prospective studies that compare MTX with other drugs used in the systemic treatment of psoriasis, including ciclosporin and biologic agents such as adalimumab and infliximab,3–6 the variability in its use in terms of dose and dose escalation makes it difficult to reach any consensus on MTX in routine clinical practice. While the recent literature includes rigorous systematic reviews and expert consensus documents that have contributed recommendations on some aspects of the use of MTX in psoriasis, other topics have not been covered.7,8
The objective of this consensus conference was to produce a set of recommendations on various aspects of the use of MTX in the treatment of moderate to severe psoriasis based on the available evidence and experience in routine clinical practice.
Consensus MethodologyA working group was formed comprising 15 dermatologists with particular expertise in psoriasis—all members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology (AEDV). The participants were all experienced in the management of MTX therapy in patients with psoriasis and authors of publications on the subject. Three members of the group acted as coordinators and the other 12 formed a panel of expert discussants whose task was to rate the validity of the proposals put forward by the coordinators. The coordinators drew up and agreed on a list of topics (Table 1) to ensure that all the questions regarding the clinical management of MTX in psoriasis considered of interest would be included. The questions were developed using the PICO methodology (patient, intervention, comparison, outcome). This set of questions was used as a framework for both the literature review and the subsequent recommendations.
Summary of Topics Considered Categorized by Area.
| Before Starting MTX Therapy | MTX Therapy | Monitoring MTX Therapy | Therapeutic Combinations with MTX | Use of MTX in Complex Clinical Situations |
|---|---|---|---|---|
| ✓ Vaccination ✓ Tuberculosis ✓ Conception and pregnancy ✓ Safety profile | ✓ Pre-treatment screening ✓ Starting dose and dose adjustment ✓ Route of administration ✓ Response time ✓ Use in children ✓ Use in psoriatic arthritis ✓ Use in other indications ✓ Folate supplementation | ✓ Patients without risk factors | ✓ Combination with NB-UV-B ✓ Combination with biologic therapy | ✓ Patients with liver disease ✓ Older patients ✓ Patients with cancer ✓ Patients with hepatitis B infection ✓ Patients with HIV infection |
Abbreviations: MTX, methotrexate; NB-UV-B, narrowband UV-B therapy.
We performed a literature search of the MEDLINE and Cochrane Library databases using an initial timeframe of 5 years (articles published since May 2009) and expanding these limits if no results were obtained with the filters used. Filters were used to restrict findings to clinical practice guidelines, consensus documents, systematic reviews, meta-analyses, and clinical trials in humans published in English or Spanish. The general keywords used were “psoriasis” and “methotrexate.” More specific terms were added relating to each section of the consensus, such as “methotrexate/contraindications,” “tuberculosis/diagnosis,” “pregnancy,” “test dose”, “subcutaneous administration,” “folic acid,” and “hepatopathy.” The search was completed with articles selected directly by the coordinators and the addition of the Summary of Product Characteristics for MTX.9
Drafting of RecommendationsThe content relating to each question was extracted from the publications selected and the quality of each source was assessed using the checklists developed by the Scottish Intercollegiate Guidelines Network (SIGN).10 Evidence-based recommendations for the use of MTX were then drafted for each of the topics on the predefined list.
Consensus ProcessOn the basis of the literature review and the draft document, the coordinators drew up a questionnaire containing the proposed recommendations, which was sent for appraisal to each one of the discussants. The scale of percent agreement was defined as follows: consensus, when at least 85% of the panel agreed with the recommendation; majority, when between 66% and 84% of the panel agreed; and discrepancy, when percent agreement was under 66%. Each expert indicated whether or not he or she was in agreement with each recommendation. Panelists could also comment on the recommendations.
After the first results were pooled and analyzed, the recommendations on which consensus had not been reached were sent to the panelists again together with any comments made in the first round. They were submitted to a second round of voting. After the second round of voting, the working group met in person to discuss remaining discrepancies and to vote once again on the recommendations using an anonymous, electronic voting system.
A document containing the agreed recommendations was then prepared. The recommendations were categorized by level of evidence (LE) and grade of recommendation (GR) using the modified SIGN system in accordance with the Spanish National Health guideline development manual (Manual metodológico de elaboración de guías del Sistema Nacional de Salud).11 For details see Tables s1 and s2 in the supplementary material.
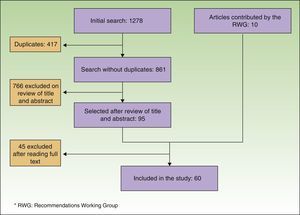
Consensus RecommendationsLiterature Search and Development of RecommendationsThe literature search identified 861 references. Of these, 95 were selected on the basis of a review of title and abstract. Ten other articles, selected manually, were added to the list: some because they were published after the literature search had been performed and others because the expert panel deemed them to be important but they had not been identified by the initial search strategy. After the complete text of all the articles had been read, the content of 60 of them was used (Fig. 1).
After the 2 rounds of consensus voting and the subsequent meeting, 27 recommendations had been validated (percent agreement ≥ 85%), 15 unanimously (100%) (Table 2).
Summary of Consensus Recommendations.
| No. | Recommendation | % A | LE/GRa |
|---|---|---|---|
| Before Starting Methotrexate Therapy | |||
| 1 | In patients with moderate to severe psoriasis, the patient's vaccination status should be reviewed before treatment with MTX is started, as required with other systemic immunosuppressants. | 92% | IV/D |
| 2 | MTX therapy is not recommended in patients who have been immunized with a live attenuated virus vaccine in the preceding 3 months. | 85% | ✓ |
| 3 | Although the evidence indicates that the risk of reactivation of latent TB is low, screening is recommended in all patients before treatment with MTX in line with the common strategy of pretreatment screening when systemic therapy is required. | 100% | IV/D |
| 4 | Latent TB infection in a patient on MTX should, as a general rule, be treated in the same way as in a patient on any other systemic immunosuppressive agent. | 86% | ✓ |
| 5 | As a general rule, men on MTX should not father a child during treatment and should wait until 3 months after discontinuing the therapy before attempting to conceive. However, in light of the lack of strong scientific evidence on the interaction of MTX with spermatogenesis, a patient who expresses a desire to father a child can continue on a low-dose regimen after he has been appropriately informed and his decision has been noted in his medical record. | 100% | 4/D 2+/D |
| 6 | Women should wait 3 to 6 monthsb after discontinuation of MTX before becoming pregnant. | 92% | 4/D |
| 7 | MTX can be prescribed in most of the situations described as “relative contraindications,”19,25 but in such cases individualized monitoring of treatment is required and the potentially higher risk should be taken into account. | 100% | ✓ |
| 8 | The presence of cardiovascular risk factors is not a contraindication to MTX therapy. | 93% | ✓ |
| Starting Methotrexate Therapy | |||
| 9 | The recommended starting dose for MTX in psoriasis is 10-20mg/wk if the patient has no risk factors for adverse effects (impairment of renal function, risk of hepatotoxicity, drug interactions). If any of these risk factors are present, treatment can be started at lower doses (5-7.5mg/wk), with clinical and laboratory assessment at 1 month. It should, however, be noted that the minimum effective dose of MTX in most patients is 7.5mg/wk, except when renal clearance of the drug is reduced. | 100% | ✓ |
| 10 | In most patients, the therapeutic dose is considered to be 15mg/wk, and the maximum dose is 20 to 25mg/wk. If clinical tolerance and laboratory test results are acceptable 3 to 4 weeks after start of treatment, the dose should be adjusted to the level considered to be the optimum therapeutic dose for the patient. | 100% | ✓ |
| 11 | In most patients, the maximum dose that can achieve a response is believed to be 20mg/wk. However, an increase to 25mg/wk can be considered in some cases. | 86% | 1++/B |
| 12 | In patients taking MTX for psoriasis who have achieved a satisfactory response, the dose can be tapered gradually by 2.5 to 5mg every 2 to 4 months until the optimum dose for the patient has been identified. | 100% | ✓ |
| 13 | Parenteral administration is the preferred route of administration in patients who may be susceptible to dosing errors or noncompliance and in patients at risk for gastrointestinal intolerance. Parenteral administration is also appropriate when the clinical response is inadequate in a patient receiving the full dose orally (≥15mg/wk). | 100% | 4/D |
| 14 | Based on the available evidence, it would appear reasonable to wait 12 to 16 weeks after the patient starts the optimum dose of MTX before considering the possibility of treatment failure. It should be noted, nonetheless, that most patients who respond to treatment with MTX show improvement before week 8. | 100% | ✓ |
| 15 | MTX (0.3mg/kg/wk) is a treatment option for psoriasis in children, although the evidence is scant. | 92% | ✓ |
| 16 | Folate supplementation is recommended in patients on MTX and is warranted by a potential reduction in adverse effects and the fact that the impact of such therapy on treatment response is low. | 85% | 4/D |
| 17 | Since there is no evidence to support a preference for the use of either folic acid or folinic acid, the choice will depend on the preferences of the specialist. Folic acid does have the additional advantage from the point of view of efficiency that it is cheaper and has pharmacokinetic advantages in some patient subpopulations. | 100% | 1++/A |
| 18 | The recommended regimen for folate supplementation is 5 to 15mg/wk for folic acid or 15mg/wk for folinic acid, taken 24-48hours after administration of MTX. | 86% | 4/D |
| Monitoring Methotrexate Therapy | |||
| 19 | In patients without risk factors, an appropriate laboratory workup should be performed in the initial weeks of treatment. If liver enzymes are elevated (<3 times the normal limit), close monitoring is recommended, with repeat analysis within 2-4 weeks and dose reduction if required. If liver enzymes are persistently higher than this level (>3 times the upper level of normal), the appropriateness of MTX therapy should be reconsidered. | 100% | IV/D |
| 20 | Despite their limitations, noninvasive methods—including serial measurement of PIIINP and transient elastography—are useful tools for monitoring MTX-induced hepatotoxicity. | 86% | IV/D |
| Therapeutic Combinations with Methotrexate | |||
| 21 | In appropriate candidates, MTX can be administered in combination with infliximab or adalimumab to prevent the development of antidrug antibodies and/or to enhance the pharmacokinetics of the biologic agent. | 100% | 2++/B |
| Methotrexate in Complex Clinical Situations | |||
| 22 | Precautions should be taken when MTX is prescribed to a patient with hepatic risk factors or liver disease: the starting dose should be lower than those proposed as standard for most patients; the initial low dose should be increased gradually to identify the lowest effective dose; factors that could determine MTX toxicity—including concomitant therapy and alcohol consumption—should be controlled; and monitoring must be rigorous (laboratory testing and any other tests deemed necessary). | 100% | ✓ |
| 23 | Clearance of MTX is less efficient in older patients, who are more likely to experience serious adverse effects. In these patients, lower doses should be used for both the initial and maintenance regimens. The initial doses proposed are between 5 and 7.5mg/wk. The dose should then be adjusted depending on the clinical course and response in each case. | 100% | ✓ |
| 24 | Although the therapeutic objective will be the same in older patients as in other age groups, safety should be prioritized in this age group because of the increased frequency of certain risk factors (renal failure, drug interactions) associated with serious side effects. | 86% | ✓ |
| 25 | Treatment with MTX is a good option for patients with a history of malignancy, as are topical treatment, phototherapy, and acitretin. The dermatologist should assess the risks and benefits of the therapy on a case-by-case basis and obtain the patient's informed consent before starting MTX. | 100% | 4/D |
| 26 | MTX therapy is not recommended in patients with chronic hepatitis B virus infection because of the risk of reactivation of the infection and the hepatotoxic effects of the drug. | 100% | 4/D |
| 27 | As there is insufficient evidence to determine the safety of MTX therapy in patients with HIV infection, the decision to use MTX to treat psoriasis in these patients should be individualized and the risk-benefit assessed. | 85% | 3/D |
Abbreviations: A, level of agreement; GR, grade of recommendation; LE, level of evidence; MTX, methotrexate; PIIINP, amino-terminal propeptide of type iii collagen; TB, tuberculosis; ✓, recommended best practice based on the clinical experience of the consensus guideline development group.
Details of the methodology used to grade evidence and recommendations can be found in Tables 1 and 2 of the supplementary material.
1. In patients with moderate to severe psoriasis, the patient's vaccination status should be reviewed before treatment with MTX is started, as required with other systemic immunosuppressants (IV/D).
Since there is evidence that the response to vaccination is suboptimal in patients on MTX, it is important to check the immunization status of any patient who is to receive this drug.12 Moreover, although the increase in the risk of infections in patients on MTX is not entirely clear in psoriasis, it is possible that these patients will eventually require additional medication or an increased dose. For this reason, Wine-Lee et al.12 recommend that all patients with moderate to severe psoriasis should undergo a study to ascertain vaccination status, including: vaccination and disease history for Haemophilus influenzae, tetanus, pertussis, varicella zoster virus, hepatitis A and B virus, human papillomavirus, influenza, Neisseria meningitidis, and Streptococcus pneumoniae. The study should include serology for hepatitis.
2. MTX therapy is not recommended in patients who have been immunized with a live attenuated virus vaccine in the preceding 3 months (✓).
MTX therapy is contraindicated in patients who have recently been vaccinated with live, attenuated vaccines.12,13 However, the optimum interval has not been clearly established. The National Psoriasis Foundation guidelines do not specify the length of the interval recommended between vaccination and the start of treatment.13 Wine-Lee et al.12 base their recommendation for an appropriate washout period on the fact that 1 to 3 months are needed for immunity to return to normal.14 Therefore, the expert panel agreed on a washout period of 3 months, similar to the interval specified for other immunomodulatory treatments.
Tuberculosis3. Although the evidence indicates that the risk of reactivation of latent tuberculosis (TB) is low, screening is recommended in all patients before treatment with MTX in line with the common strategy of pretreatment screening when systemic therapy is required (IV/D).
The treatment of psoriasis with immunosuppressive drugs may be associated with an increased risk of reactivation of TB. While there is only scant evidence for the reactivation of latent TB during treatment with MTX,15–17 a complete assessment is advised for any patient with psoriasis in whom systemic treatment is indicated. As several authors have suggested, the need for a complete study arises because of the chronic nature of the condition and the variability of response to treatment.18,19 Consequently, pretreatment assessment should include standard procedures that may later be required if the patient is switched to treatment with other immunomodulatory drugs. Screening also provides a baseline reference if pulmonary complications develop. Patients can be screened using either the Mantoux test (repeated to identify a booster effect when necessary)19 or in vitro techniques based on interferon-γ release assay (IGRA) since the available evidence does not favor the use of either of these 2 procedures in patients treated with MTX. If the test result is positive, a chest radiograph must be obtained to rule out the presence of active infection.
4. Latent TB infection in a patient on MTX should, as a general rule, be treated in the same way as in a patient on any other systemic immunosuppressive agent (✓).
According to Bogas et al.,20 MTX therapy is probably not associated with a significant increase in the incidence of TB,21 so that such infection in patients on MTX should be managed the same way as it is managed in the general population. Other authors specify that patients at risk for TB should be screened, should receive chemoprophylaxis with isoniazid, and should be monitored closely during MTX therapy,22 with particular attention to the risk of hepatotoxicity due to isoniazid, which is increased by the concomitant use of MTX.
Conception and pregnancy5. As a general rule, men on MTX should not father a child during treatment and should wait until 3 months after discontinuing the therapy before attempting to conceive (4/D).
However, in light of the lack of strong scientific evidence on the interaction of MTX with spermatogenesis, a patient who expresses a desire to father a child can continue on a low-dose regimen after he has been appropriately informed and his decision has been noted in his medical record (2+/D).
There are conflicting guidelines on the recommended attitude to the continuation of MTX therapy in men intending to father a child. While some authors defend the need for a 3-month washout period before conception after withdrawal of MTX—despite the paucity of evidence—others suggest that the dosage regimen can be maintained without modification. Some review articles and the American and Spanish guidelines recommend that men should avoid fathering a child during MTX therapy and for at least 3 months after cessation of treatment,18 given that a spermatogenesis cycle lasts some 74 days. By contrast, Weber et al.23 emphasize the lack of evidence supporting the hypothesis that the risk of adverse events during pregnancy is higher when the father has followed a low-dose regimen of MTX (≤30mg/wk), asserting that it is, therefore, unnecessary to subject men to a 3-month MTX-free period prior to attempting to father a child, an opinion shared by some other authors.18,24
6. Women should wait 3 to 6 months after discontinuation of MTX before becoming pregnant (4/D).
It has been suggested that, in women, the interval between withdrawal of MTX and possible conception should be between 1 ovulatory cycle and 3 months,19,25 although the Summary of Product Characteristics specifies a 6-month washout period.9 In the opinion of Carretero et al.,19 although the length of the interval between the end of treatment and pregnancy has not been established, women should avoid pregnancy for at least 1 ovulatory cycle after withdrawal of treatment given the characteristics of MTX. The European guidelines25 state that MTX is absolutely contraindicated during both pregnancy and breastfeeding, specifying a washout period of 3 months for both sexes.
Safety profile7. MTX can be prescribed in most of the situations described as “relative contraindications,”19,25 but in such cases individualized monitoring of treatment is required and the potentially higher risk should be taken into account (✓).
There are several relative contraindications to MTX therapy (Table 3).19,25 However, in view of the scant evidence supporting these restrictions, MTX may be administered in most of these situations, bearing in mind the potentially higher risk of adverse effects and ensuring close monitoring and follow-up of such patients.
Relative Contraindications to Methotrexate Therapy.
| Recent vaccination with a live vaccine |
| Impaired renal or hepatic function |
| History of hepatitis |
| Ulcerative colitis |
| Gastritis |
| Congestive heart failure |
| Old age |
| Lack of adherence to therapy |
| Drug Interactions |
| Active desire to have a child for women of childbearing age and for men |
| Diabetes mellitus |
| Obesity |
| Hyperlipidemia |
| Hypoalbuminemia |
| Folate deficiency |
| History of malignancy |
8. The presence of cardiovascular risk factors is not a contraindication to MTX therapy (✓).
In recent years, evidence has emerged of an association between psoriasis and various cardiovascular risk factors (metabolic syndrome, obesity, hypertension, dyslipidemia, and type 2 diabetes mellitus) and, therefore, with cardiovascular disease.26 Prodanovich et al.27 concluded that treatment with MTX in patients with psoriasis or rheumatoid arthritis significantly reduces the risk of vascular disease as compared to patients not treated with MTX. The reduction was more notable in the patients who received a low cumulative dose of MTX, and was further reduced by the concomitant use of folic acid.27 There is also evidence that MTX can reduce the risk of myocardial infarction,28 and that the use of tumor necrosis factor (TNF)-α inhibitors in severe plaque psoriasis is accompanied by an improvement in the biomarkers for cardiovascular risk.29 Although the evidence is insufficient to reliably support an assertion that MTX has a positive impact on cardiovascular risk factors, it can be concluded that it is not contraindicated in patients with this profile.
Starting Methotrexate TherapyPretreatment screening required with MTXPretreatment assessment begins with the patient's medical history, a physical examination, and a laboratory workup. Based on Kalb et al.,18 the panel agreed that the pretreatment workup should include at least the following: complete blood count, kidney function tests, liver function tests, serology for hepatitis B and C, screening to rule out latent or active TB infection, and a pregnancy test in women of childbearing age.
Initial dose and dose adjustment9. The recommended starting dose for MTX in psoriasis is 10 to 20mg/wk if the patient has no risk factors for adverse effects (impairment of renal function, risk of hepatotoxicity, drug interactions). If any of these risk factors are present, treatment should be started at a lower dose (5-7.5mg/wk), with clinical and laboratory reassessment at 1 month. It should, however, be noted that the minimum effective dose of MTX in most patients is 7.5mg/wk, except when renal clearance of the drug is reduced. (✓).
The dose range for MTX in the studies reviewed was 5 to 15mg/wk as an initial dose, with a low starting dose in most cases to minimize adverse effects. However, the practice of starting treatment with a low dose is historical rather than evidence-based.30 The European guidelines suggest a low starting dose (5-10mg/wk) 25; the American guidelines state that there are no standardized maximum or minimum doses and that the range is from 7.5 to 25mg13; the Spanish guidelines accept 7.5mg/wk as a starting dose, but note that better results are obtained with higher doses19; and a systematic review recommends starting with 5 to 10mg/wk for the first week.8 Notwithstanding these suggestions, the expert panel agreed that a reasonable starting dose in a patient with no risk factors could be 10mg/wk or higher given that higher doses achieve better results and are not associated with any increase in adverse effects.
10. In most patients, the therapeutic dose is considered to be 15mg/wk and the maximum dose is 20 to 25mg/wk. If clinical tolerance and laboratory test results are acceptable 3 to 4 weeks after start of treatment, the dose should be adjusted to the level considered to be the optimum therapeutic dose for the patient (✓).
According to Paul et al,8 the therapeutic dose of MTX is between 15 and 25mg/wk, although the experts considered that a dose of 15mg/wk is usually sufficient in most cases. In the studies reviewed, maximum doses ranged between 20 and 25mg/wk and in some cases as high as 30mg/wk. In a systematic review, the maximum recommended dose was 25mg/wk.8 On the basis primarily of accumulated clinical evidence rather than the findings of controlled studies, the authors of the European guidelines concluded that the maximum dose should not exceed 30mg/wk.25 The expert panel considered that a period of approximately 1 month is generally required to achieve the optimal dose for each patient.
11. In most patients, the maximum dose that can achieve a response is believed to be 20mg/wk. However, an increase to 25mg/wk can be considered in some cases (1++/B).
According to a subanalysis of the CHAMPION study, if an acceptable response has not been achieved by week 12 with a dose of 20mg/wk of MTX, the likelihood of improving the response by increasing the dose to 25mg/wk is very low.5 In view of this finding, it does not seem logical in most cases to prescribe a dose higher than 20mg/wk; however, some patients may benefit from higher doses (25mg/wk), overweight or obese patients for example.
12. In patients taking MTX for psoriasis who have achieved a satisfactory response, the dose can be tapered gradually, reducing it by 2.5 to 5mg every 2 to 4 months until the optimum dose for the patient is identified (✓).
There were very few references in the literature to dose reduction in MTX therapy. Paul et al.8 concluded that there is no solid evidence supporting any specific strategy for escalating or de-escalating treatment. Consequently, the proposal on reducing the dose is based on the experience of the expert panel.
Route of administration13. Parenteral administration is the preferred route of administration in patients who may be susceptible to dosing errors or noncompliance and in patients at risk for gastrointestinal intolerance. Parenteral administration is also an acceptable option when the clinical response is inadequate in a patient receiving the full dose orally (≥15mg/wk) (4/D).
There are no studies comparing oral and parenteral administration in patients with psoriasis.7 However, studies in patients with rheumatoid arthritis show that parenteral administration improves the effectiveness of the drug.31,32 A systematic review recommends starting with oral treatment unless the response is inadequate, gastrointestinal intolerance is an issue, or there is a risk of the patient not adhering to the treatment regimen.8 In such cases, parenteral administration with the same dose can be considered. On the other hand, subcutaneous administration improves bioavailablility when the dose is 15mg/wk or higher.33 Therefore, it may be beneficial to use a subcutaneous rather than oral route of administration for such regimens. The expert panel considered oral and parenteral administration of MTX to be equally acceptable options and agreed that one or the other might be preferred depending on the circumstances, the dose used, or the patient's preference.
Timing of response to treatment14. Based on the available evidence, it would appear reasonable to wait until the patient has received the optimum dose of MTX for 12 to 16 weeks before considering the possibility of treatment failure. Nevertheless, It should be noted that most patients who respond to treatment with MTX will show improvement before week 8 (✓).
Owing to the great variability in the way MTX is prescribed, most of the recommendations are based on clinical practice and expert experience. This circumstance is reflected in the studies reviewed, in which a certain imprecision and variation can be observed in the assertions made. However, an analysis of the evidence indicates that most responders will show signs of a good response at 8 weeks and will respond by week 12. After week 12, it is unlikely that a higher response rate will be achieved. According to the results of the CHAMPION study, even with a conservative regimen only 5% of the patients who have not responded in the early weeks will respond later.5 Even so, as per the NICE guidelines, it is reasonable to wait until week 12 to assess treatment if the dose reached is at least 15mg/wk, and up to a maximum of 16 to 24 weeks in the case of patients on lower doses.34 Consequently, the expert panel considered that 8 weeks might be an insufficient interval for some patients and increased this period to 12 or even 16 weeks in certain cases.
Using MTX in children15. MTX (0.3mg/kg/wk) is a treatment option for psoriasis in children, although the evidence is scant (✓).
There is very little information available on the treatment of psoriasis in children, and the existing information consists of expert opinion and data from case series. A systematic review concluded that MTX is an effective treatment option for moderate to severe psoriasis in children, with good tolerance in the short term.35 Similarly, a European consensus document drafted using the Delphi method concluded that MTX is an appropriate treatment in severe cases of guttate psoriasis in children, recommending a dose of 0.3mg/kg/wk regardless of the age of the child but without exceeding the maximum standard dose of 22.5mg/wk.36
Methotrexate in the management of psoriatic arthritisToday, disease-modifying antirheumatic drugs (DMARD) are considered to be a second-line treatment after nonsteroidal anti-inflammatory drugs for most patients with moderate peripheral psoriatic arthritis, and MTX is the DMARD of choice in this setting.37
Methotrexate in other indicationsThere is very little evidence available on the use of MTX in other indications related to psoriasis, such as onychopathy38,39 pustular and erythrodermal forms of the disease, and palmoplantar pustulosis.
Folate supplementation16. Folate supplementation is recommended in patients on MTX and is warranted by a potential reduction in adverse effects and the fact that the impact of such therapy on treatment response is low (4/D).
The use of high doses of folinic acid to reverse serious episodes of acute MTX toxicity led to the evaluation of combining folate supplementation with a low-dose regimen of MTX.40 Since folic acid reduces the hepatotoxicity and can normalize elevated liver transaminases,40–46 its use in combination with MTX also ensures greater tolerability of MTX at doses above 15mg/wk.42,43 Moreover, folic acid supplementation is inexpensive, has almost no adverse effects according to most studies, and does not compromise the efficacy of MTX.47 It would therefore appear to be a reasonable adjuvant therapy in patients with moderate to severe psoriasis being treated with MTX.
17. Since there is no evidence to support a preference for the use of either folic acid or folinic acid, the choice will depend on the preferences of the specialist. Folic acid does have the additional advantage from the point of view of efficiency that it is cheaper and has pharmacokinetic advantages in some patient subpopulations (1++/A).
There is no clear evidence on whether it is preferable to use folic acid or folinic acid as an adjuvant therapy with MTX, although some dermatologists prefer folic acid.8 When the results of adding folic acid or folinic acid were assessed in a systematic review, the results were similar for both drugs. In terms of efficiency, however, folic acid is the preferred option because of its lower cost.48 Moreover, only folic acid, and not folinic acid, inhibits aldehyde oxidase and could contribute to increasing MTX concentrations in fast metabolizers, thereby enhancing the effectiveness of treatment.49
18. The recommended regimen for folate supplementation is 5 to 15mg/wk for folic acid and 15mg/wk for folinic acid, taken 24 to 48 hours after administration of MTX (4/D).
Several strategies are suggested in the Spanish Guidelines19; a daily dose of 5mg of folic acid (except on the days when MTX is taken); 15mg/wk of folinic acid taken 24 to 48hours after administration of MTX; or 5mg taken 24 to 48 hours after administration of MTX if the patient does not require a higher dose. One systematic review recommends a supplement of 5mg/d of folic acid for 1 to 3 days starting 48 hours after administration of MTX, preferably orally.7 Other guidelines recommend the same regimen, and specify which days the patient should receive each drug to avoid confusion.8
Monitoring Methotrexate TherapyPatients without risk factors19. In patients without risk factors, an appropriate laboratory workup should be performed in the initial weeks of treatment. If liver enzymes are elevated (but<3 times the upper limit of normal), close monitoring is recommended, with repeat analysis within 2 to 4 weeks and dose reduction if required. If liver enzymes are persistently higher than this level (> 3 times the upper limit of normal), the appropriateness of MTX therapy should be reconsidered (IV/D).
Following the recommendations of the American Academy of Dermatology guidelines13 and the consensus document on the use of MTX in psoriasis by Kalb et al.,18 in patients without risk factors hepatotoxicity should be monitored by evaluating liver chemistries. Both these guidelines recommend performing a liver biopsy only when aspartate aminotransaminase (AST) levels are persistently elevated over a 12-month period or if serum albumin falls below the normal range (in patients with normal nutritional status) in a patient with well-controlled disease. At this time, however, the expert panel considered that the best course of action in patient with persistent abnormal laboratory test results is to consider withdrawal of MTX and switching to another therapy before performing a liver biopsy.
20. Despite their limitations, noninvasive methods—including the serial measurement of amino-terminal propeptide of type iii collagen (PIIINP assay) and transient elastography—are useful tools for monitoring MTX-induced hepatotoxicity (IV/D).
The authors of some guidelines have proposed biopsy within 2 to 6 months of starting MTX and a repeat biopsy after every 1 to 1.5g cumulative dose if the patient has 1 or more risk factors for hepatotoxicity.13,18 However, other authors advocate the use of less invasive techniques, such as serial measurement of PIIINP levels and vibration-controlled transient elastography.18,25,50 The constraints of medical practice require a feasible and realistic protocol, and frequent laboratory workups or liver biopsies are not a reasonable option in the routine monitoring of patients, particularly since the latter is a procedure entailing considerable cost and risk. Indeed, biopsies are not part of routine monitoring in dermatological practice and should only be considered in exceptional cases, since they will usually lead to a change of therapeutic strategy. For this reason, it is essential to carefully select appropriate candidates for MTX therapy. In a patient with relative risk factors for MTX therapy (obesity, diabetes mellitus, elevated transaminases), the recommendations will be similar to those for a patient without risk factors, that is, careful management of dose and treatment monitoring; moreover, MTX should be seen as a provisional treatment option.
Therapeutic Combinations with MethotrexateMethotrexate in combination with narrowband-UV-B phototherapyThe combination of MTX with narrowband-UV-B phototherapy (NB-UV-B) is more effective than NB-UV-B alone because it facilitates reductions in both dose and exposure.25,50–52 Although this combination is theoretically associated with a higher risk of nonmelanoma skin cancer, the risk with the combination of MTX and NB-UV-B is lower than with MTX and psoralen plus UV-A.52 However, long-term studies are needed.
Combination of MTX with biologic therapy21. In appropriate candidates, MTX can be administered in combination with infliximab or adalimumab to prevent the development of antidrug antibodies and/or to enhance the pharmacokinetics of the biologic agent (2++/B).
Immunogenicity reduces the therapeutic response to TNF inhibitors, particularly in the case of infliximab and adalimumab. A recent systematic review concluded that the use of concomitant immunosuppressant therapy, especially MTX, attenuates the impact of antidrug antibodies on the efficacy of TNF blockers.53 Adding low doses of MTX is especially useful in the case of infliximab, and improves efficacy.19,54 Adding MTX to a regimen of adalimumab could also improve clinical outcomes in both psoriasis and psoriatic arthritis.19,55 In patients with moderate to severe psoriasis, combination therapy with etanercept and MTX has an acceptable safety profile and has been shown to be more effective than treatment with etanercept alone.56
Using Methotrexate in Complex Clinical SituationsPatients with liver disease22. Precautions should be taken when MTX is prescribed to a patient with hepatic risk factors or liver disease: the starting dose should be lower than those proposed as standard for most patients; the initial low dose should be increased gradually to identify the lowest effective dose; factors that could determine MTX toxicity—including concomitant therapy and alcohol consumption—should be controlled; and monitoring must be rigorous (laboratory testing and any other tests deemed necessary) (✓).
Avoiding therapy with MTX is recommended in patients with risk factors for hepatotoxicity. Prolonged use of MTX can induce fibrosis and cirrhosis, and in people with psoriasis these complications may not be preceded by symptoms or abnormal laboratory test results. Patients with non-alcoholic steatohepatitis also have a higher risk of hepatotoxicity with MTX.57
Older patients23. Clearance of MTX is less efficient in older patients, who are more likely to experience serious adverse effects with this drug. In older patients, lower doses should be used for both initial and maintenance regimens. The initial doses proposed are between 5 and 7.5mg/wk. The dose should then be adjusted taking into account the clinical course and response in each case (✓).
The dose required to control severe psoriasis is lower in patients aged over 50 years because of age-related changes in metabolism and decreased renal clearance in this population.58 In elderly patients, effective control of psoriasis can be achieved with a low-dose regimen of MTX,59 and adequate disease control has been observed with as little as 2.5mg/wk in patients aged 80 years or older.60
24. Although the therapeutic objective will be the same in older patients as in other age groups, safety should be prioritized in this age group because of the increased frequency of certain risk factors (renal failure, drug interactions) associated with serious side effects (✓).
While the overall toxicity of MTX is not any higher in patients aged over 65 than in the younger population, gastrointestinal, hepatic, and hematologic adverse effects are more frequent this age group50 and particular care is needed when treating this population.
Patients with cancer25. Treatment with MTX is a good option for patients with a history of malignancy, as are topical treatment, phototherapy, and acitretin. The dermatologist should assess the risks and benefits of the therapy on a case-by-case basis and obtain the patient's informed consent before starting MTX (4/D).
The decision to use of MTX in patients with lymphoreticular malignancies and solid tumors, including malignant melanoma, will depend on the time that has elapsed since diagnosis of the tumor. Depending on the particulars of each case, MTX therapy may or may not be preferred over other treatments, such as acitretin, phototherapy, and topical corticosteroids.57 The treatment of psoriasis in patients with cancer should be tailored to the individual case, and patients should be informed about the lack of complete certainty regarding the effects on recurrence of the therapies available.
Patients with hepatitis B infection26. MTX therapy is not recommended in patients with chronic hepatitis B virus infection because of the risk of reactivation of the infection and the hepatotoxic effects of the drug (4/D).
There are better alternatives for the treatment of psoriasis in patients with chronic hepatitis B infection57 because of the high risk of hepatotoxicity in patients with chronic and even latent forms of viral hepatitis who are carriers of the hepatitis B virus.61,62 Moreover, several cases have been reported of reactivation of hepatitis B caused by MTX in hepatitis B surface antigen-positive patients and in some patients who were surface antigen-negative but anti-HBc-positive.63–68
Human immunodeficiency virus-seropositive patients27. As there is insufficient evidence to determine the safety of MTX therapy in patients with HIV infection, the decision to use MTX to treat psoriasis in these patients should be individualized and the risk-benefit assessed (3/D).
Although the recommendation not to use MTX in HIV-positive patients dates back to the earliest cases in which the drug was administered to such patients, other subsequent studies did not confirm the increased risk.69,70 However, since no clinical trials have assessed the efficacy and safety of MTX in this population, the decision to use low doses of MTX in this setting should be individualized and be accompanied by strict monitoring of the disease.71
DiscussionAlthough MTX is fully integrated into the therapeutic armamentarium for moderate to severe psoriasis with many years of accumulated clinical experience, there is a surprising lack of scientific evidence relating to many aspects of its use. The present article offers an overall view of the available evidence complemented by the opinion of dermatologists experienced in the use of methotrexate in this setting. The aim was to provide answers to most of the difficult questions encountered in the management of MTX therapy in patients with psoriasis. The high levels of agreement (above 85%) achieved on all of the recommendations and the fact that the decision on more than half of the recommendations was unanimous clearly demonstrates that they represent the opinion of the experts consulted.
While the literature contains several reviews dealing with the use of MTX in psoriasis, few of them involved expert opinion integrated using participatory methodological procedures.3,4,15 Based on a systematic review of the literature, Mountadié et al.7 focused on the doses that should be used, the route of administration, and adverse effects, particularly the risk of fibrosis. In an article not unlike the present one, Paul et al.8 complemented their review of the literature with expert opinion using the Delphi method and a panel of 66 dermatologists, focusing on the same aspects.9,10
Both of those articles recommended a similar dosing strategy for starting treatment with MTX: an initial dose ranging from 5 to 10mg/wk, with rapid escalation until the therapeutic dose is reached. In the present study, however, the panel found no justification for using a low starting dose when the patient had no risk factors relating to safety, given the clear relationship reported in the literature between the dose of MTX and treatment efficacy.
Likewise, the authors of both those review articles were in agreement on the appropriateness of using noninvasive techniques to monitor potential liver damage, despite the technical limitations of such techniques at this time. The primary objective is to discontinue the use of liver biopsy—a very rare procedure in routine clinical practice today—as a requirement in patients treated with MTX for long periods.
The present article also offers a practical view on aspects that have rarely been reviewed. These include the precautions that should be taken with respect to vaccination and TB prophylaxis, which are similar to those applied in other systemic treatments, and the considerations that affect different age groups, which represent a challenge in the management of the severe forms of psoriasis.58 The route of administration is also discussed. While oral MTX is generally considered in the literature to be the standard prescription, subcutaneous administration could offer advantages in terms of tolerance and effectiveness (although to date this has only been demonstrated in other inflammatory diseases) and could offer improved safety when there is risk of dosage error. These advantages must, however, be evaluated in the context of the higher cost of treatment.
The present study has certain limitations. First, the methodology used in the review was not comprehensive, thereby reducing the number of articles identified by the literature search. This limitation could generate potential biases in the analysis of the results. The methodology used did, however, ensure the identification of the studies with the highest methodological quality. Another limitation inherent in all consensus documents on the use of MTX in psoriasis is the lack of quality studies that can provide the data necessary for evidence-based recommendations. Consequently, most of the proposed recommendations have a low level of evidence or are based on expert opinion. However, given this circumstance, which has also represented a challenge to the authors of other reviews, recommendations generated through expert consensus acquire particular importance for the clinician interested in the management of MTX therapy.
Finally, for each of the recommendations, the expert dermatologists were only permitted to offer an affirmative or negative vote, unlike the methodology used in the Delphi method. However, the high level of agreement achieved shows that the proposed recommendations do adequately reflect the opinion of the panelists. In conclusion, the present consensus document proposes recommendations relating to the use of MTX that are based on the available evidence and the clinical experience of experts. The aim is to offer a useful tool to clinicians involved in the management of patients with moderate to severe psoriasis.
FundingThis work was made possible by the financial support of Gebro Pharma, S.A. However, no employees of Gebro Pharma participated in the scientific discussion or played any role in the development or revision of the manuscript, which was drafted independently by the scientific committee.
Conflicts of InterestGebro Pharma facilitated the meeting of the working group and provided technical and methodological support. However, none of its employees participated in the development or production of the scientific material, the discussions, or the manuscript. José Manuel Carrascosa has received speaking fees from Gebro Pharma.
The authors acknowledge the technical and methodological support of Verónica Albert of GOC Networking for her support in implementing the methodology used in the study.
Please cite this article as: Carrascosa JM, de la Cueva P, Ara M, Puig L, Bordas X, Carretero G, et al. Metotrexato en psoriasis moderada-grave: revisión de la literatura y recomendaciones de experto. Actas Dermosifiliogr. 2016;107:194–206.