Graft-vs-host disease (GVHD) is a multisystem disease that arises as a complication of allogeneic hematopoietic stem cell transplant. It is due to recognition of the recipient's tissues by immune cells from the donor. The skin and mucous membranes are the organs most commonly affected. GVHD is classified as acute or chronic depending on the pathophysiology and clinical presentation. Acute GVHD typically presents with the triad of rash, diarrhea, and hyperbilirubinemia, and treatment is based on systemic corticosteroid and immunosuppressant therapy. The cutaneous manifestations of chronic GVHD are divided into sclerodermiform and nonsclerodermiform, and the mucous membranes and skin appendages may also be affected. The diagnosis is mainly clinical, but skin biopsy can help in doubtful cases. Treatment can be topical, systemic, or physical, depending on the size, site, and depth of the lesions and the involvement of other organs.
La enfermedad injerto contra huésped (EICH) es una enfermedad multisistémica que aparece como complicación de un trasplante de progenitores hematopoyéticos alogénico. Se basa en el reconocimiento de tejidos del receptor por parte de la inmunidad heredada del donante. La piel y las mucosas son los órganos más frecuentemente afectados. Se clasifica en aguda y crónica, en función de su fisiopatología y presentación clínica. La forma aguda se manifiesta típicamente con la tríada de exantema, diarrea e hiperbilirrubinemia, y el tratamiento se basa en el uso de corticoides e inmunosupresores sistémicos. Las manifestaciones cutáneas de la forma crónica se dividen en esclerodermiformes y no esclerodermiformes. Puede afectar también a mucosas y faneras. El diagnóstico es fundamentalmente clínico, aunque en casos dudosos la biopsia cutánea puede ayudar a confirmarlo. El tratamiento puede ser tópico, sistémico o físico, en función de la extensión, localización, profundidad de las lesiones y afectación de otros órganos.
Graft-vs-host disease (GVHD) is a multisystem complication of allogeneic hematopoietic stem cell transplantation (HSCT), which is the only curative treatment for certain blood diseases. HSCT replaces a patient's ineffective stem cells with a healthy donor's effective ones, derived from bone marrow, peripheral blood, or umbilical cord blood.1 In spite of measures to prevent HSCT from developing, the incidence of this complication remains high.1 Any organ can be affected, but the skin and mucous membranes are most often involved (in 20% to 70% of cases). Cutaneous lesions are also the most quickly noticed and easiest biopsied.
GVHD contributes in great measure to the morbidity and mortality associated with allogeneic HSCT. If the blood tumors that motivated HSCT recur, the most frequent cause of death is this complication, which continues to be the main obstacle to more widespread use of a cell replacement procedure that offers the only curative treatment for a variety of diseases of the blood and other organs.1
Pathophysiology and ClassificationGVHD develops when the donor's immune cells recognize the recipient's tissues as foreign as a result of interaction between the recipient's antigen-presenting cells and the donor's mature T-cells,1 leading to immune dysregulation that triggers inflammation and the destruction of the recipient's cells. This alloimmune and autoimmune disorder results in an immunodeficient state that affects both quality of life and survival.2,3 Although the phenomenon of GVHD has negative consequences for the patient, there is also a beneficial effect for patients with blood tumors given that primary tumor cells are targeted as well as healthy tissues (the concept of antitumor vigilance).4 For this reason, when treating GVHD a balance is sought. The goal is to control the phenomenon but not abolish it completely so that the potent antitumor effect is preserved.
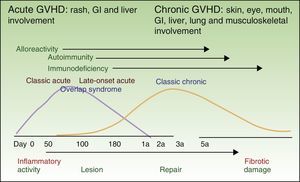
GVHD is classified as either acute or chronic, traditionally distinguished by a cutoff of 100 days after HSCT. Today, however, they are defined by pathophysiological mechanisms and clinical presentation5 (Table 1). The inflammatory changes that predominate in the initial phases are mainly due to a reaction to the graft. Autoimmune reactions and immunodeficiency develop in later phases, and over time the reparative process leads to fibrotic tissue damage (Fig. 1). Although inflammation and repair are dynamic and overlapping processes, patients do not necessarily present signs of both and they are not explained by the same pathophysiological mechanisms. Therefore, the 2 processes are studied separately.
GVHD Classification.
| Types | Time Elapsed Since HSCT | Signs and Symptoms of Acute GVHDa | Signs and Symptoms of Chronic GVHDb |
|---|---|---|---|
| Acute GVHD | |||
| Classic | ≤100 d | Yes | No |
| Persistent, recurrent, or late-onset | >100 d | Yes | No |
| Chronic GVHD | |||
| Classic | No time limits | No | Yes |
| Lichenoid | Earlier | ||
| Sclerodermoid | Later | ||
| Overlap syndrome | Yes | Yes | |
Adapted from Filopovich et al.5
Abbreviations: GVHD, graft-vs-host disease; HSCT, hematopoietic stem cell transplant
Acute GVHD is described by a 3-phase model consisting of 1) a conditioning regimen involving damage to keratinocytes in a proinflammatory environment, 2) induction of antigens against the donor's T cells by the host's dendritic cells, followed by activation of the donor-derived T cells, and 3) activation of a type-1 helper T cell (Th1) response that leads to necrosis of keratinocytes.6
The pathophysiology of chronic GVHD involves both allogeneic and autoimmune reactions. Once the thymus is damaged in the conditioning regime and/or by acute GVHD, the organism's tolerance of its own cells is impaired. In the complex response that develops, CD4+ and CD8+ T cells, regulatory T cells, and B cells participate in producing autoantibodies. Th1, Th2 and Th17 responses produce proinflammatory cytokines that cause fibrosis and organ failure.7–9 High rates of elevated autoantibody titers (of antinuclear antibodies, antibodies to double-stranded DNA, and anti-smooth muscle antibodies) have been observed in patients with chronic GVHD, and although this finding is unrelated to specific organ involvement, it has been associated with higher risk for extensive, sclerotic chronic GVHD in some studies.10–12
Acute Graft-vs-Host DiseaseThe patient's environment must be studied during the first stage after HSCT to guide diagnosis and treatment. In the 100 days after a transplant, the patient undergoes intense, induced immunosuppression. The immune system is gradually reconstituted in a process regulated by various hematopoietic cell series once the graft takes root. The patient is also given multiple myeloablative, immunosuppressant, and antiinfective agents. Infectious complications and drug-related adverse reactions are frequent.
The most important risk factors for acute GVHD are HLA disparity, advanced donor or recipient age, donor alloimmunization (prior transfusions, pregnancy), use of peripheral blood as the source of progenitor hematopoietic cells, and the number of T cells in the graft.6
Symptoms, Staging, and ClassificationAlthough GVHD is a multisystem disease, the skin and mucosal tissues are the most commonly affected organs. Cutaneous signs and symptoms typically develop early; therefore, they often hold the key to diagnosis. Liver and intestinal involvement is the second most common source of signs. Thus, skin rash, elevated bilirubin levels, and diarrhea form the characteristic clinical triad.13
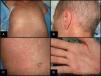
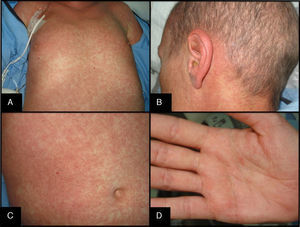
Skin symptoms may present as the dysesthesia, itching, erythema, or edema that develop into a progressive morbilliform or generalized folliculotropic rash that is found mainly on the trunk, spreads centrifugally, and becomes confluent. A rash on the palms, soles and skin behind the ear is typical (Fig. 2). In severe eruptions, blisters (dermal-epidermal detachment) may form. Also typical is oral, genital, nasal, or ocular mucositis.
GVHD is staged (0 to 4) according to signs and symptoms and the percentage of body surface area (BSA) involved.13 Liver involvement is also staged from 0 to 4 according to blood and intestinal bilirubin levels and the severity of diarrhea.
Acute GVHD is considered classic if onset occurs within 100 days of HSCT, persistent if it lasts beyond 100 days, recurrent if it resolves but reappears after 100 days, and late-onset if symptoms start after 100 days (Table 1 and Fig. 1).5
Diagnosis and Differential DiagnosisThe diagnosis of acute GVHD is based on clinical evidence of the characteristic triad (skin rash, elevated bilirubin levels, and diarrhea), although all organs are not necessarily involved.
In the early phase following HSCT, other common complications that also present with rash must be considered. Adverse drug reactions affecting the skin and infectious rashes (often viral) must be included in the differential diagnosis.14 Palmar-plantar erythrodysesthesia, syringometaplasia, and toxic epidermal necrolysis are relatively common toxic drug reactions in these patients and must be distinguished from GVHD. When viral rashes develop after HSCT, the usual culprits are reactivated cytomegalovirus, Epstein-Barr virus, herpes simplex virus types 1 and 2, varicella zoster virus, and human herpesvirus types 6, 7 and 8.
The diagnosing physician should bear in mind the clinical context and timing of lesion appearance, drugs taken and other systemic manifestations. Nevertheless, differentiating these processes is often dauntingly difficult.
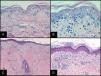
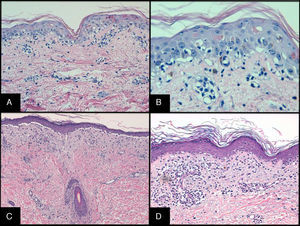
Histopathology and Pathologic StagingHistopathologic evaluation of a skin biopsy can aid the diagnosis of acute GVHD, but there is no specific finding that confirms the diagnosis.15 It is helpful to include hair follicles in the biopsy. Characteristic findings are vacuolar interface dermatitis with necrotic keratinocytes surrounded by lymphocytes (Fig. 3). When the condition is advanced, dermal-epidermal detachment and subepidermal blisters can be seen.
Histopathology of cutaneous GVHD.
A and B, Acute GVHD. Hematoxylin and eosin (H&E), original magnification ×200 and H&E, original magnification×400, respectively. Interface dermatitis with necrotic keratinocytes surrounded by lymphocytes (lymphocyte satellitosis). C and D, Chronic GVHD. H&E, original magnification×100 and H&E, original magnification×200. Interface dermatitis with dermal-epidermal detachment and follicular involvement.
Histopathologic observations are graded from 0 to 4 (similarly to clinical signs) according to the degree of dermal-epidermal involvement.16
The same obstacles are encountered in histologic diagnosis as in clinical diagnosis: essentially acute GVHD must be distinguished from toxic drug reactions and infectious rashes. Certain histopathologic features can be helpful. Whether or not eosinophil counts are useful for differential diagnosis is controversial.15,17 A finding of adnexal involvement would favor a diagnosis of GVHD, whereas the presence of spongiosis, a dense inflammatory infiltrate, and extravasation of blood would point to a drug reaction. Enzyme-linked immunosorbent assay findings have demonstrated elafin overexpression throughout the epidermis,18 and this biomarker might therefore prove useful for the differential diagnosis of drug reactions, in which only the granular layer would stain.
Chronic GVHDThe onset of chronic GVHD occurs once the first phase (the 100 days after HSCT) is over. The mean lag period is between 4 and 6 months.19 In the later phase, immunosuppression is less marked but still present as a result of both immunosuppressants and the effects of HSCT itself. Patients are also being exposed to several drugs, whose side effects may appear immediately or with the passing of time.
The risk factors for developing chronic GVHD are as follows: earlier acute GVHD, advanced donor or recipient age, a female recipient of a graft from a male donor, an unrelated donor or HLA disparity, use of progenitor hematopoietic cells derived from peripheral blood (which confer greater risk than cells from bone marrow, which in turn confer greater risk than cells from umbilical cord blood), a diagnosis of chronic myeloid leukemia, and infusion of white blood cells from the donor.6
Factors related to poor prognosis are as follows: progressive onset, involvement of more than 50% of the BSA at diagnosis, thrombocytopenia (platelet count<100 000 cells/μL), lung involvement (bronchiolitis), and multiple organ involvement.20
It is important to mention that strategies for lowering the incidence of acute GVHD have not affected the incidence of the chronic form.21
Symptoms, Staging, and ClassificationChronic GVHD can appear as an extension of acute GVHD (the progressive chronic form), or it can follow a disease-free period (the quiescent form) or develop without prior GVHD signs (the de novo form).2,3
The classic presentation (with the distinctive signs and symptoms of chronic GVHD but with no limits on timing) is distinguished from overlap syndrome (where acute and chronic signs and symptoms are both present).5
Chronic GVHD is a multisystem disease that can affect any organ, either one or several at a time. The involvement of the skin and oral mucosa is most common.22 Other affected organs in decreasing order of frequency are the liver, the eyes (dry-eye syndrome), the intestines, and the lungs.
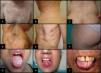
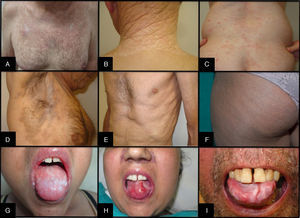
Cutaneous signs and symptoms affecting skin, mucosal tissue, or adnexa can vary greatly. Sclerodermiform and nonsclerodermiform manifestations are distinguished.23,24 The first type comprises (from superficial to deeper signs) lichen sclerosus et atrophicus, morphea, and fasciitis. The second includes lichen planus, poikiloderma, and keratosis pilaris. Other less common clinical presentations are panniculitis, changes in pigmentation and sweating, erythema, rash, pruritus, ulceration, vitiligo and alopecia areata, among others25 (Fig. 4). Lichenoid lesions tend to appear earlier and may evolve into sclerotic lesions, although not all such lesions have a lichenoid derivation, nor do all lichenoids evolve in this way.14 It is not unusual for lesions to appear on skin previously damaged by burns, radiotherapy, or herpes infection (isotopic response) or where there has been repeated trauma (isomorphic response).23
Although nail involvement is of relatively little importance, it affects half of patients with chronic GVHD. Manifestations might take the form of nail dystrophy, thickening, thinning onycholysis, vertical striae, or pterygium. Similarly, the scalp is often involved, through alopecia (scarring or nonscarring), although the root causes are usually multiple (e.g., chemotherapy, radiotherapy, hormone changes, deficits, GVHD).23
Oral mucosa may be affected by oral lichen planus, hyperkeratosis, sclerosis, xerostomia, mucocele, mucosal atrophy, pseudomembranes, and ulcers, among other conditions23 (Fig. 4).
Genital mucosal involvement is less common and the patient does not usually ask about it spontaneously. Direct questioning and a complete physical examination is therefore required to rule out this manifestation, which is often an obstacle for sexual relations. Genital lichen planus, vaginal stenosis, erosions, fissures, or ulcers may be present.23
Table 2 lists the cutaneous and mucosal signs and symptoms of chronic cutaneous GVHD.
Signs and Symptoms of Chronic GVHD.
| Diagnostic Features | Distinctive Features | Other Features | Features Common to Both Acute and Chronic GVHD | |
|---|---|---|---|---|
| Skin | Poikiloderma | Depigmentation | Changes in sweating | Erythema |
| Lichen planus-like features | Maculopapular rash | |||
| Sclerotic changes | Pruritus | |||
| Morphea | ||||
| Lichen sclerosus | ||||
| Mouth | Lichen planus | Xerostomia | Gingivitis | |
| Hyperkeratosis | Mucoceles | Mucositis | ||
| Sclerosis | Mucosal atrophy | Erythema | ||
| Pseudomembranes | Pain | |||
| Ulcers | ||||
| Genitalia | Lichen planus | Erosions | ||
| Vaginal stenosis | Fissures | |||
| Ulcers | ||||
| Nails | Dystrophic nails | |||
| Brittle nails, striations | ||||
| Onycholysis | ||||
| Pterygium | ||||
| Nail loss | ||||
| Scalp | Alopecia | Fine, uneven or dull hair | ||
| Papulosquamous lesions | Early graying | |||
Chronic GVHD is graded in order to indicate severity and evaluate response to treatment. Grading systems are available for specific organs or to evaluate GVHD globally according to type and number of organs involved and severity. The most widely used system in clinical practice, because assessment is rapid and simple, is that of the US National Institutes of Health (NIH).26–28 A severity level of 0 to 3 assigned for each affected organ takes into consideration the impact on function. Table 3 shows the NIH grading system for the skin and oral and genital mucosa. Other more complex scales are available for use in clinical trials, although comparative studies have not demonstrated they are superior to the NIH's for determining disease activity, the physician and patient's perception, or relation to survival.29,30
US NIH Grading System for Chronic GVHD.
| Score | Skin | Mouth | Genital tract |
|---|---|---|---|
| 0 | No signs or symptoms | ||
| 1 | Skin signs on ≤18%of the BSA, without sclerosis | Mild symptoms, not significantly limiting oral intake | Mild symptoms with no impact on sexual function |
| 2 | Skin signs on 19% to 50% of the BSA, with superficial sclerosis | Moderate symptoms with partial limitation of oral intake | Moderate signs, with discomfort on examination |
| 3 | Skin signs on ≥50% of the BSA Deep sclerosis Impaired mobility, ulceration, or severe itching | Severe symptoms with major limitation of oral intake | Advanced signs (vaginal stenosis, labial agglutination, severe ulcers) and severe pain during coitus |
Abbreviations: BSA, body surface area; GVHD, graft-vs-host disease; NIH, National Institutes of Health.
The signs and symptoms of chronic GVHD are classified by type as follows31:
- -
Diagnostic: sufficient to establish a diagnosis of chronic GVHD without resort to other tests
- -
Distinctive: insufficient alone for establishing a firm diagnosis without complementary tests or evidence of lesions on other organs
- -
Other features, or unclassified ones: also insufficient for establishing a diagnosis
- -
Common features: seen in both chronic and acute GVHD
Table 2 summarizes skin and mucosal signs and symptoms according to these categories.
A diagnosis of chronic GVHD requires the following findings31:
- -
The presence of at least 1 diagnostic sign or symptom, or the presence of at least 1 distinctive sign or symptom plus histologic confirmation
- -
The exclusion of other possible diagnoses
- -
Differential diagnosis with acute GVHD
It is important to remember that not all skin lesions in patients who have undergone HSCT are signs of GVHD. Like other patients, graft recipients can also develop drug reactions, inflammatory lesions, infections, benign or malignant tumors, or other conditions. Therefore, the physician must consider the correlation of clinical and pathologic features and timing when diagnosing any skin lesion.
Histopathology and Pathologic StagingA skin biopsy is recommended to confirm diagnosis, although it is not absolutely necessary if the patient has a sign or symptom considered diagnostic. Biopsies are suggestive but not specific for either chronic or acute GVHD, although a histologic report that findings are “compatible with” or “diagnostic for” GVHD is sufficient to confirm the diagnosis if the patient has manifestations classified as distinctive.31 The minimum histologic criteria that must be met for diagnosis are the presence of apoptosis in the basement membrane of the epidermis or the external root sheath of a hair follicle or the acrosyringium. These findings may be accompanied by a lichenoid infiltrate, vacuolar changes or lymphocytes surrounding necrotic keratinocytes (lymphocyte satellitosis). There are additional specific criteria according to type of chronic GVHD32 (Fig. 3).
TreatmentProphylaxis and Life SupportFollowing HSCT, all patients should keep skin well hydrated, undertake moderate exercise, and avoid sun exposure by using sun blockers and sun glasses.23
Dry mouth and genitalia are very common symptoms of GVHD and can be alleviated through topical and oral medications33:
- -
Dry mouth syndrome: artificial saliva and oral pilocarpine (5mg every 6–8h)34
- -
Dry genitals: humectants and hormonal therapy.
Given that acute GVHD is a multisystem disease that appears in immunocompromised patients, and customarily presents during hospitalization for HSCT, the treating physician is usually a hematologist. The first line of therapy is methylprednisolone, and if there is no response, a second drug (e.g., antithymocyte globulin, mycophenolate mofetil, anti-tumor necrosis factor agents, or sirolimus) can be added, using departmental regimens and a tailored approach.35 However, the main approach to acute GVHD is to prevent it, through the use of corticosteroids, ciclosporin, and/or methotrexate.36
Topical treatments for skin manifestations of GVHD are inadequate. Phototherapy may be useful in cases that are resistant to conventional treatment.37–42
Chronic GVHDDermatologists play a more important role in managing and treating chronic GVHD. Certain issues must be considered before choosing an approach. Once again, we should recall the multisystem nature of the disease and always investigate the involvement of other organs. It is also important to distinguish sclerodermiform and nonsclerodermiform types, verify depth (epidermal, dermal, or subcutaneous involvement), note the extension of manifestations (localized vs generalized), and consider location when evaluating cutaneous signs. Manifestations involving more than 50% of the BSA predict a poor prognosis.43
Topical TreatmentsWhen skin manifestations are localized, epidermal, or dermal, topical preparations can be used. Corticosteroids33 and tacrolimus44,45 can be useful; the second is particularly appropriate for the face and skin folds and as a way to reduce corticosteroid use. Topical medications can also be used on the oral and genital mucosa.46,47 Different corticosteroids are prescribed according to their potency at the affected site and lesion depth.
Formulations that are useful inside the mouth are 0.1% triamcinolone acetonide (mouthwash or oral adhesive gel), 0.05% clobetasol (oral adhesive gel), and 0.1% tacrolimus (ointment); a ciclosporin mouthwash can be useful in patients on long-term corticosteroids.48
Systemic TreatmentsSystemic therapy should be considered whenever topical treatments targeting the skin have failed (i.e., signs or symptoms do not improve or become worse), multiple organs or a high percentage of the BSA is involved, or cutaneous or fascial sclerosis has been demonstrated.23,31 The patient's concomitant conditions, general state of health, disease phase, and risk of infection must be factored into the decision.
Oral prednisone at a dose of 1mg/kg is the first line of treatment. There is no standardized rescue treatment if prednisone fails. The choice of alternatives should be individualized according to clinical manifestations of GVHD and other patient-related factors. Sirolimus, mycophenolate mofetil, rituximab, imatinib, azathioprine, thalidomide, methotrexate, and others can be used. Of all these alternatives, imatinib mesylate at a dose of 100 to 200mg/d is the most specific for sclerodermiform cutaneous GVHD.49–51 In all cases in which systemic treatment is being considered, the hematologist and other specialists treating the patient should be consulted and consensus sought.
Physical Therapies- 1.
Phototherapy. Psoralen plus UV-A (PUVA), UV-A1, UV-B, and narrow-band UV-B phototherapies have been used.52 These alternative therapies can be useful for patients with generalized lesions that are refractory to other measures, or they can be used as a corticoid-sparing strategy.53 The choice of type of phototherapy is based mainly on the depth of lesions and the patient's concomitant conditions. Sclerotic lesions respond best to PUVA, whereas superficial lesions respond to narrow-band UV-B treatment. A finding of ocular GVHD would contraindicate PUVA. The dermatologist should take care to investigate any photsensitizing drugs (especially voriconazole) the patient might be taking as well as a positive antinuclear antibody test result.54
- 2.
Extracorporeal photopheresis. This therapy is particularly appropriate for cutaneomucosal forms of GVHD and patients who have become dependent on corticosteroids or have hepatic or pulmonary involvement.55,56 The technique requires placement of a central venous catheter for apheresis of white blood cells and platelets from whole blood. After treatment with PUVA, the concentrated collected blood is reinfused. The procedure, which is repeated twice a week for several months, is time-consuming. Although few side effects develop during treatment, risk of infection and sepsis is present.
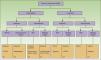
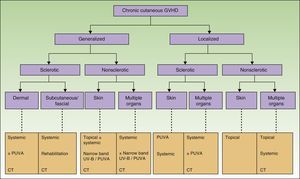
Figure 5 shows a flow chart of available treatments for chronic cutaneous GVHD.
Flow chart for treatment of chronic cutaneous GVHD.
GVHD refers to graft-vs-host disease; CT, clinical trial; PUVA, psoralen plus UV-A; NB-UV-B, narrow-band UV-B.
Adapted from Hymes et al.23
No validated, standardized criteria are available for measuring response to therapy for chronic cutaneous GVHD. This situation complicates the management of this condition and the design of clinical trials.
Disease activity must be measured at 2 time points in order to quantify and compare response. It is important to assess skin lesions, depth of involvement (epidermis, dermis, or subcutaneous tissue), and BSA expressed as a percentage.
Definitions for complete response (all lesions have disappeared), partial response (only improvement can be perceived), and progression (the condition has worsened) have been proposed.31 However, the definitions are subjective and reproducibility is a problem. Furthermore, clinical manifestations are varied (dry mouth, sclerotic cutaneous lesions, etc.) and the fact that some are irreversible makes it difficult to standardize response criteria.
Outlook for the FutureAcute GVHDAcute GVHD continues to represent the main limiting factor affecting the use of allogeneic HSCT. Biomarkers for early risk assessment and diagnosis are needed.57 The treatment of acute GVHD is less than optimal, and we need new approaches to prevention and management that preserve the antitumor effects of the graft.
Chronic GVHDThe pathogenesis of chronic GVHD is still poorly understood. Interest in the role of cytokines in the development of clinical signs and their influence on severity has increased given that greater understanding might suggest potential therapeutic targets.58
Assessing response to chronic GVHD therapy is one of the greatest challenges we face. Available grading systems are unable to assess disease extension and severity or measure the effect of treatment. There are no clinical or laboratory findings that can distinguish active chronic GVHD from residual fibrosis.
Nor is there a laboratory test for a biomarker that can predict risk of developing chronic GVHD, response to treatment, or survival. Such biomarkers are essential for identifying risk and initiating treatment early as well as for closely monitoring response. They can also be helpful for measuring disease activity and the balance between chronic GVHD and the antileukemia effect of the graft. Biomarkers are currently being developed and their use validated, but none have been applied routinely in clinical practice.59,60
ConclusionsGVHD is a multisystem disease that can affect any organ and should therefore be managed through a multidisciplinary approach.
Improving our understanding of GVHD and validating biomarkers of disease activity can help us maximize the therapeutic potential of HSCT and minimize the risk of GVHD.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Pau Alonso and Conrad Pujol of the hematology section of the pathology department of Hospital Universitario y Politécnico de la Fe in Valencia, Spain.
Please cite this article as: Ballester-Sánchez R, Navarro-Mira M, Sanz-Caballer J, Botella-Estrada R. Aproximación a la enfermedad injerto contra huésped cutánea. Actas Dermosifiliogr. 2016;107:183–193.