Lupus erythematosus tumidus (LET), a form of cutaneous lupus erythematosus that was described some years ago, has begun to receive more attention in the past decade as many published studies have helped to define the particularities of this subtype.The clinical features of LET include the absence of changes on the surface of the epidermis (such as erosion, atrophy, scaling, or follicular plugging) and the lack of scarring on resolution.Because flares are easily induced on exposure to sunlight, eruptions tend to occur in episodes.The diagnosis of LET remains difficult, however, as we continue to debate such issues as the classification of this disease, certain of its microscopic features, and the differential diagnosis of LET in relation to such entities as polymorphic light eruption or Jessner lymphocytic infiltration.
El lupus eritematoso túmido (LET) es una forma de lupus eritematoso cutáneo (LEC) ya descrita en la literatura hace años. Sin embargo, no ha sido hasta la última década en que esta entidad ha generado un mayor interés, dando lugar a la publicación de numerosos trabajos que han permitido caracterizarlo como un subtipo de LEC con unos rasgos particulares. Dichos rasgos son, desde el punto de vista clínico, la ausencia de alteraciones en la superficie epidérmica (erosión, atrofia, descamación y taponamiento folicular) y la curación sin dejar cicatriz. Las lesiones son, por otra parte, fácilmente inducibles por la fotoexposición, por lo que suelen cursar a brotes. Sin embargo, algunas cuestiones acerca del LET, como son su clasificación, ciertas características microscópicas y el diagnóstico diferencial con otras entidades, como la erupción polimorfa lumínica o la infiltración linfocitaria de Jessner, siguen siendo objeto de controversia a día de hoy y dificultan el diagnóstico de estos pacientes.
The term lupus erythematosus tumidus (LET) was first used in a publication by Gougerot and Burnier1 in 1930 to describe smooth, infiltrated, erythematous lesions with no desquamation or other superficial changes observed in 5 patients. However, the condition received little attention in the following years, with the publication of only a few isolated cases,2–6 and it was not included in the classification of cutaneous lupus erythematosus (CLE) proposed by Gilliam7 in the 1970s, which contemplated 3 main clinical types: chronic CLE (CCLE), which included discoid lupus erythematosus (DLE) as the most important subtype; subacute CLE (SCLE); and acute CLE (ACLE). The absence of LET from that classification no doubt contributed not only to its consideration as a rare form of lupus, but also to its relative obscurity. In the past, patients with LET were typically diagnosed with CCLE or with what some authors called “papular lesions,” despite clinical and histopathological descriptions consistent with LET.8
In the past decade a number of authors have shown renewed interest in LET and have made an effort to characterize the condition. It has thus come to be defined as a subtype of CLE with its own clinical, prognostic, and microscopic features. It has also become clear that the frequency of LET is very likely underestimated9,10; in fact, there is a widespread view that it may even be more prevalent than classic DLE. However, a number of issues remain, including its classification, certain microscopic characteristics, and the differential diagnosis with other conditions; as we shall see, some authors have even questioned whether it is a true lupus. All these aspects will be discussed in detail in the following sections.
EpidemiologyThe frequency of LET is higher than was thought some years ago and, as commented above, most authors agree that LET is by no means a rare form of lupus. Although there are no studies that provide firm data about its prevalence or incidence, some 250 cases have been described in the literature to date.11 As most of these reports have been published within the past 10 years, this figure represents a considerable number of cases.
Unlike SCLE and ACLE, LET affects men and women in roughly equal proportion.9–12
The mean age at onset of the disease is between the 36.4 and 38.5 years, similar to that of DLE.11,12 It should be noted that there have also been reports of cases in children, with the same clinical and histopathological features as in adult patients.13
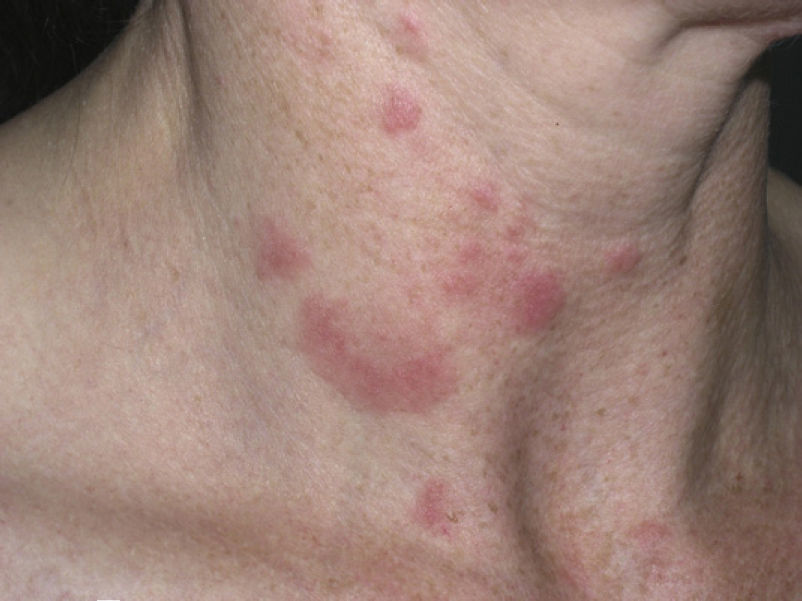
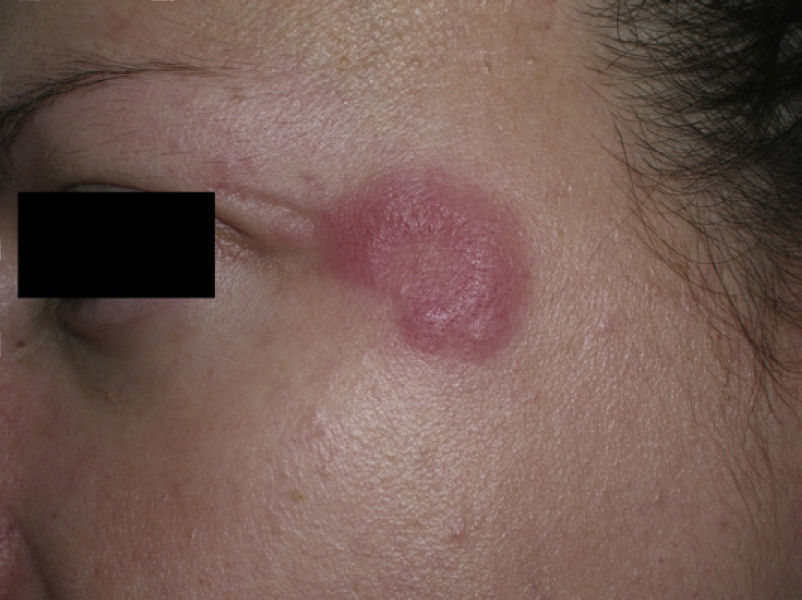
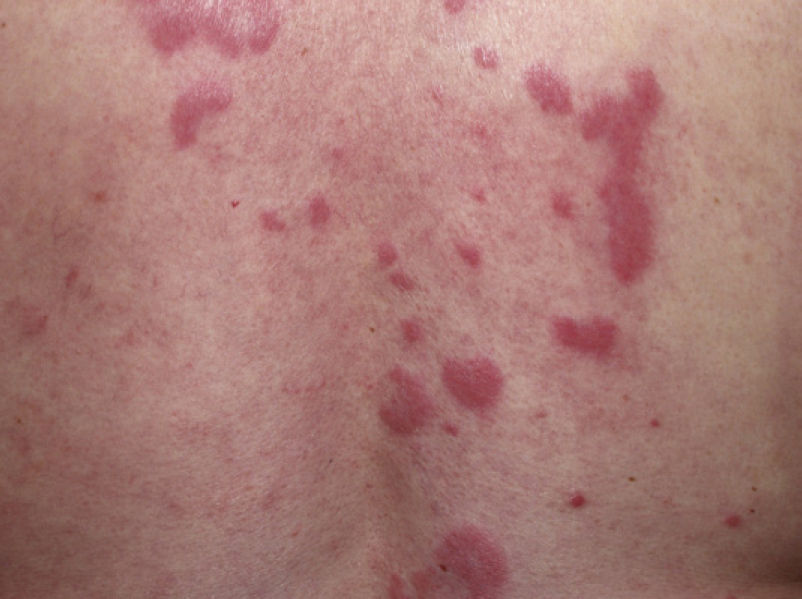
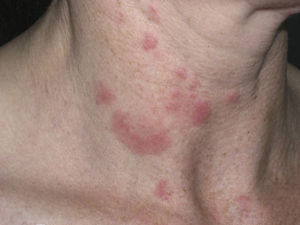
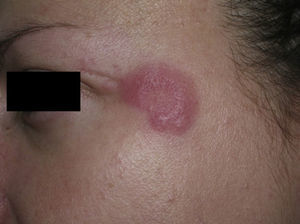
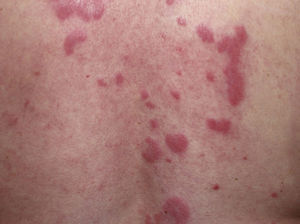
Clinical PresentationThe manifestations of LET consist of erythematous papules, plaques, or annular lesions with a succulent appearance; lesions develop mainly on sun-exposed areas such as the neckline, shoulders, face, and arms. The features that differentiate LET lesions from those of DLE and SCLE are the absence of desquamation, follicular plugs, or atrophy (Figs. 1–3); in addition, they heal without leaving a scar or hypopigmentation.9–12,14–16 The lesions tend to appear in crops, almost always related to exposure to the sun, mainly during the spring and summer. It is important determine the course of lesions after exposure to the sun, as this helps to differentiate LET from polymorphous light eruption. In LET, the lesions do not appear immediately, but rather after a latency period that varies between 24hours and several weeks.11,12,17,18 Moreover, they tend to persist throughout the summer and even into the autumn. Below, we look in detail at certain aspects of photosensitivity in LET and the possible etiological and pathogenic factors involved.
Another distinctive feature of LET is that it is rarely associated with antinuclear (ANA), anti-Ro, anti-La, or anti-DNA antibodies.9–12,14–16 Blood tests also show few alterations, and it is uncommon to observe cytopenias or abnormalities of renal function. These findings, together with the low frequency of the systemic complications typical of systemic lupus erythematosus (SLE), indicate that the systemic behavior of LET is very similar to that of DLE.
Photosensitivity in Lupus Erythematosus Tumidus and Theories on its Etiology and PathogenesisLET is characterized by marked photosensitivity, and this has been demonstrated experimentally in a number of photobiological studies. Based on that research, LET is considered to be the most photosensitive form of CLE, more so even than SCLE. In LET, 70% to 76% of lesions are photoinduced,11,15,17,18 a higher proportion than in SCLE (62% to 63%).11,17 For many years, photosensitivity in CLE has been considered to be very closely linked to the presence of autoantibodies in the blood, principally anti-Ro antibodies.19,20 Although no direct relationship has been demonstrated in vivo between photosensitivity and the presence of these antibodies,21 their involvement in at least some types of lupus erythematosus appears very likely. Another suggestive finding that supports this theory is the appearance of skin lesions in neonatal lupus linked to the presence in the infant's blood of anti-Ro antibodies passed from the mother.22
However, the marked photosensitivity of LET, a disease not typically associated with the presence of ANA, anti-Ro antibodies, or anti-La antibodies in the blood, means that we must consider alternative mechanisms that might give rise to these lesions. The results of recent research point to the involvement of a particular cell type, the plasmacytoid dendritic cells (PDC) and their product, type I interferons (IFNs α and β), as a key elements in the etiology and pathogenesis of the lesions of lupus. Their presence has been demonstrated in biopsies of the skin lesions of SLE,23–26 SCLE, and DLE,23–25,27 as well as in those of LET.23,27–29 IFN-α, together with IFN-γ produced by T lymphocytes, induces the production of a series of chemokines (CXCL 9, 10, and 11) by the structural cells of the skin (keratinocytes, endothelial cells, and fibroblasts); these chemokines, in turn, attract more T lymphocytes and PDCs. This thus sets up a positive feedback loop that amplifies the inflammatory response.23 The mechanism of activation of these PDCs is still not fully understood, but it could have its origin in cell apoptosis caused by UV radiation and the subsequent exposure of autologous DNA. In patients with SLE it has been suggested that this autologous DNA could bind autoantibodies, giving rise to immune complexes that are captured by the PDCs and activate an intracellular receptor.30 However, this mechanism cannot be extrapolated to the forms of CLE that are not associated with autoantibodies in the blood, such as DLE and LET.31 In these 2 diseases, the mechanism of activation of the PDCs could be similar to the one described by Lande et al32 in psoriasis. Those authors identified an antimicrobial peptide called LL37 that binds with autologous DNA to form complexes that are then taken up by the PDCs and trigger Toll-like receptor 9.
The lesions triggered by exposure to sunlight in LET could therefore arise when certain peptides bind to autologous DNA and form complexes that activate the PDCs, thus inducing the production of type I interferon. However, this hypothesis has not yet been confirmed.
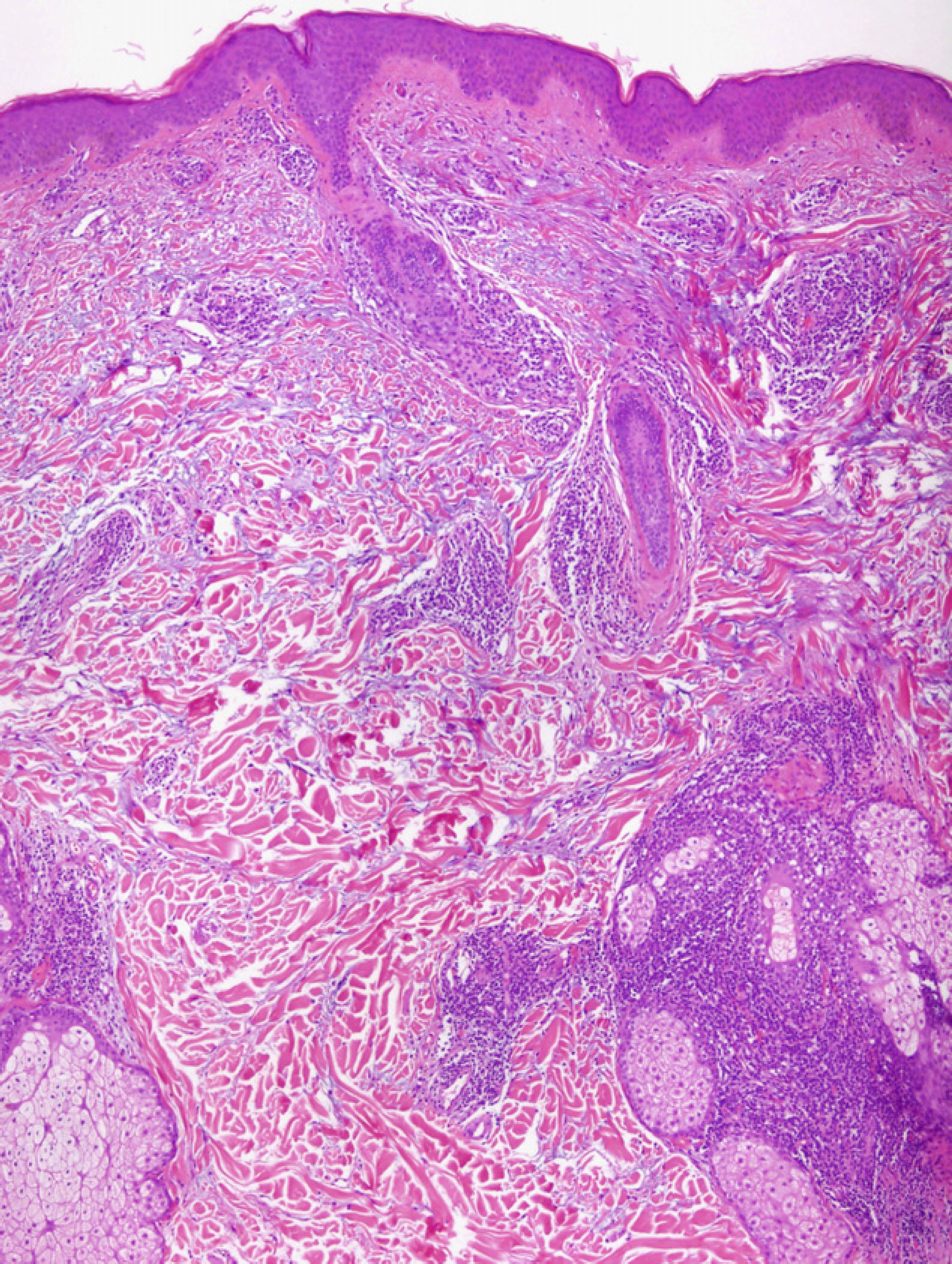
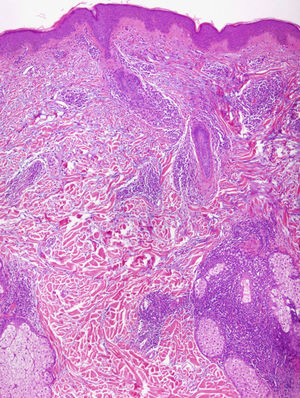
Light MicroscopyAlthough most authors agree on the clinical features and prognosis of LET, there is still debate about the histopathological features. There is consensus on the microscopic findings in the dermis, where a periadnexal and perivascular lymphocytic infiltrate is observed with interstitial mucin deposits, and several studies have reported that direct immunofluorescence does not usually reveal significant immunoglobulin or complement deposition on the basement membrane.10–12,14,16,33 There are, however, different opinions on the changes in the epidermis. According to Kuhn et al,12,33 the epidermis is intact in practically all cases, and this finding has been confirmed in other studies11,16 (Fig. 4). However, recent research has demonstrated that mild epidermal alterations, including focal vacuolization of the basal layer, hyperkeratosis, and follicular atrophy or plugging, are observed in 61% to 70% of cases; specifically, vacuolization of the basal layer is observed in 19% to 46% of biopsies.10,14 In their description of the papular lesions that we may now classify as LET, McHugh et al8 reported that biopsies from 1 group of patients presented the typical epidermal alterations of lupus, whereas in the remaining cases the epidermis was intact. In our opinion, the findings of those authors may be closer to the true picture since it is not rare in practice to encounter patients who have lesions typical of LET in whom a biopsy reveals epidermal changes. Consensus on this matter and greater definition are important because of its implications in the diagnosis of LET.
Diagnostic Criteria for Lupus Erythematosus TumidusThe diversity of opinions regarding the epidermal features of LET on light microscopy has given rise to different proposals for the diagnostic criteria of this disease; the key differences between these proposals refer to the histopathological appearance. In 2002, Kuhn et al12 established criteria that included an absence of alterations of the epidermis or at the dermal-epidermal junction.
In clinical practice, as we have mentioned above, some of the patients presenting the lesions typical of LET do not entirely fit the criteria proposed by Kuhn in that mild or moderate epidermal changes are seen in the biopsy. We therefore consider the diagnostic criteria put forward by Vieira et al10 to be closer to reality. Some later studies also accept mild or focal alterations of the epidermis when making a diagnosis of LET.11,14 In Table 1, the authors present their own adaptation of the diagnostic criteria proposed in previous publications. We have added an item on photosensitivity, which is important not for differentiation from the other forms of CLE, but to distinguish LET from certain photodermatoses, particularly polymorphous light eruption.
Diagnostic Criteria of Lupus Erythematosus Tumidus.a
| 1. Clinical presentation |
| - Erythematous papules or plaques having an urticarial or infiltrated appearance, with no surface changes (atrophy, desquamation, erosions, scabs, or follicular plugging) |
| - The lesions heal without leaving a scar |
| - The lesions tend to appear with a variable latency (>24 h) after exposure to sunlight and persist for a long period |
| 2. Biopsy findings |
| - Perivascular and periadnexal lymphocytic infiltrate in the dermis |
| - Mucin deposits in the dermis |
| - No or mild alterations of the epidermis |
| •Epidermal atrophy |
| •Vacuolar degeneration of the basal layer |
| •Follicular plugging |
| •Hyperkeratosis |
| •Thickening of the basement membrane |
| - Direct immunofluorescence usually negative |
| 3. In general, absence of the criteria of SLE |
| 4. Antinuclear, anti-Ro, anti-La, and anti-DNA antiobodies usually negative |
In summary, in clinical practice, a suspected diagnosis of LET may be established in a patient with erythematous, infiltrated papules, plaques, or annular lesions that, unlike the lesions observed in DLE or SCLE, do not present superficial alterations such as desquamation, atrophy, or erosions. The diagnosis is confirmed by biopsy showing an absence of epidermal alterations or a mild form of the epidermal changes typical of lupus erythematosus, a periadnexal and perivascular lymphocytic infiltrate, and interstitial mucin deposits in the context of a patient who, in general, does not present systemic manifestations of SLE and does not have autoantibodies.
This said, there continue to be certain problems in practice when making a diagnosis of LET. The first issue is the subjectivity of defining a mild degree of microscopic alterations of the epidermis or a clinical absence of superficial alterations. Secondly, some patients present the characteristic lesions of LET in association with autoantibodies or with the systemic manifestations of SLE,16 in which case we must consider the possibility of rare presentations of LET or exclude the diagnosis. Finally, as will be discussed below, the differential diagnosis with other conditions is not always easy.
Is Lupus Erythematosus Tumidus a True Form of Lupus?Some authors have questioned the lupic origin of LET, basing their arguments on the absence of autoantibodies, of any of the systemic manifestations of lupus erythematosus, or of an interface dermatitis in the histology study.34 Those authors consider LET to be a photodermatosis that does not form part of the spectrum of CLE. There is, however, evidence that challenges that assertion. First, as we have seen, a large proportion of cases of LET do present an interface dermatitis.8,10,14 Secondly, as the low frequency of autoantibodies and of systemic manifestations is also observed in DLE, these characteristics are not a solid argument for excluding LET from the spectrum of CLE. It should also be noted that there have been reports of patients with simultaneous lesions of LET and DLE,11,12,16 a finding that supports the view that the two conditions are different forms of the same disease. Finally, the presence of PDCs and proteins induced by IFN-α23,27–29 in the biopsies of LET lesions would suggest a common etiological and pathogenic mechanism with other forms of CLE. Thus, in our opinion, there is sufficient evidence in the literature to consider LET to be a form of lupus erythematosus.
The Place of Lupus Erythematosus Tumidus in the Classification of Cutaneous Lupus ErythematosusAs has been commented above, Gilliam's original classification did not include LET. In successive revisions and modifications of that classification, LET has been included as a subgroup of CCLE (Table 2).35,36 The main reason for this decision is the similarity between LET and CCLE in terms of prognosis and their minimal association with autoantibodies. However, as their clinical features are very different, some authors consider LET to be an independent subtype. Kuhn et al9 suggested that LET should be included as a fourth subtype called intermittent CLE (Table 3). In our opinion, this is a reasonable proposal because the essential characteristic of all the forms of CCLE is the presence of persistent lesions that leave scarring or atrophy, something that does not occur in LET. However, Kuhn et al's proposal has still not been widely accepted, and the most recent review articles continue to situate LET within the CCLE group.36
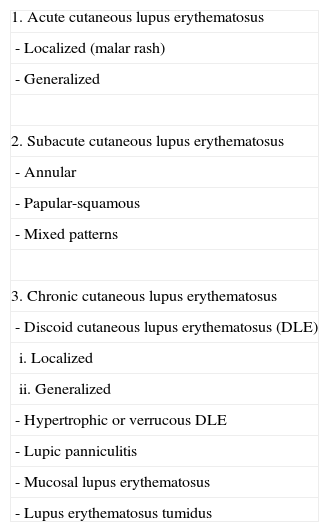
Classic Classification in Which Lupus Erythematosus Tumidus Is Included as a Subtype of Chronic Cutaneous Lupus Erythematosus.
| 1. Acute cutaneous lupus erythematosus |
| - Localized (malar rash) |
| - Generalized |
| 2. Subacute cutaneous lupus erythematosus |
| - Annular |
| - Papular-squamous |
| - Mixed patterns |
| 3. Chronic cutaneous lupus erythematosus |
| - Discoid cutaneous lupus erythematosus (DLE) |
| i. Localized |
| ii. Generalized |
| - Hypertrophic or verrucous DLE |
| - Lupic panniculitis |
| - Mucosal lupus erythematosus |
| - Lupus erythematosus tumidus |
Taken from Obermoser G et al.36
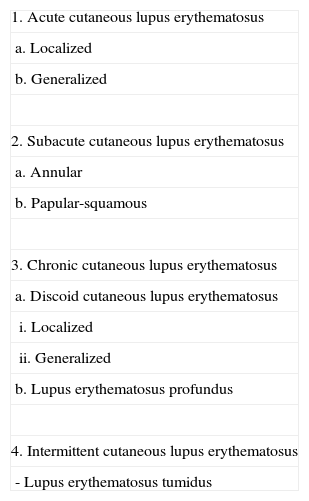
Classification of Cutaneous Lupus Erythematosus Proposed by Kuhn et al.,9 in Which Lupus Erythematosus Tumidus Is Included as a Distinct Subtype.
| 1. Acute cutaneous lupus erythematosus |
| a. Localized |
| b. Generalized |
| 2. Subacute cutaneous lupus erythematosus |
| a. Annular |
| b. Papular-squamous |
| 3. Chronic cutaneous lupus erythematosus |
| a. Discoid cutaneous lupus erythematosus |
| i. Localized |
| ii. Generalized |
| b. Lupus erythematosus profundus |
| 4. Intermittent cutaneous lupus erythematosus |
| - Lupus erythematosus tumidus |
There are 3 skin conditions that are difficult to differentiate from LET due to their clinical and microscopic similarities: Jessner lymphocytic infiltration, polymorphous light eruption, and reticular erythematous mucinosis.
Jessner lymphocytic infiltration was described in the 1950s by Jessner and Kanof37 as a condition giving rise to nonscarring lesions that mainly affect the face and that present a periadnexal and perivascular lymphocytic infiltrate on light microscopy, without alteration of the epidermis.38 As patients did not show photosensitivity and the condition did not appear to respond to treatment with antimalarials, it was considered not to be lupic in origin. However, some studies published during the following years have cast doubt on these conclusions.39,40 Studies performed in the past decade generally conclude that Jessner lymphocytic infiltration falls within the spectrum of lupus and, in particular, could be considered a form of LET. In a photobiology study in 10 patients with Jessner lymphocytic infiltration, Weber et al41 demonstrated that all the patients developed lesions after photoprovocation and observed a latency period of over 48hours before the appearance of the lesions, as occurs in all forms of CLE. Study of biopsies of the lesions revealed a perivascular and periadnexal infiltrate indistinguishable from LET, with interstitial mucin deposits and an unaltered epidermis. Their findings led them to conclude that there were no significant clinical, pathological, or photobiological differences between Jessner lymphocytic infiltration and LET. Rémy-Leroux et al42 conducted a comparative study of 32 cases of Jessner lymphocytic infiltration and 14 cases of LET. After analyzing the clinical and microscopic features and the response of the 2 groups of patients in the photobiology study, they concluded that Jessner lymphocytic infiltration and LET are indistinguishable. Based on the findings of those studies, Jessner lymphocytic infiltration is now generally considered to be a subtype of LET rather than a separate disease.
Polymorphous light eruption is a photodermatosis that gives rise to a wide variety of skin lesions, although these tend to be monomorphic in any one patient. The lesions may be vesicular or pseudovesicular, or they can take the form of papules or plaques that are difficult to differentiate from LET. In the latter case, certain differences in the clinical course of the lesions are the key to resolving the differential diagnosis. In contrast to LET, the lesions of polymorphous light eruption develop soon after exposure to sunlight and improve within a few days if there is no further exposure. Moreover, the attacks decrease in severity if exposure is maintained, as a very characteristic tolerance phenomenon develops.12 Furthermore, while the lesions in patients with LET typically persist after the summer, episodes of polymorphous light eruption are strictly limited to the spring and summer months. However, it must be said that, in contrast to the situation with Jessner lymphocytic infiltration, there are no clinical or photobiological studies that compare the features of polymorphous light eruption and LET. Microscopically, polymorphous light eruption can also be difficult to differentiate from CLE, as the 2 diseases may share certain common features, such as the perivascular lymphocytic infiltrate, vacuolar changes in the basal layer (that are mild and do not include necrotic keratinocytes), epidermal atrophy, and follicular plugging.43–45 Positive direct immunofluorescence has also been demonstrated in some cases.46 A feature that has come to be considered characteristic of this condition is edema of the papillary dermis. However, a recent study by Pincus et al47 also demonstrated this edema in biopsies from patients with CLE (SCLE and DLE). After reviewing the literature, those authors concluded that the histopathological features that differentiate CLE from polymorphous light eruption are the abundant interstitial mucin deposits, the periadnexal lymphocytic infiltrate, and the intense vacuolar degeneration of the basal layer; in the case of LET, we should focus on the first 2 features. Thus, although LET and polymorphous light eruption may be similar in some aspects, they differ in others, and should be considered to be different diseases. Wackernagel et al29 demonstrated the presence of PDCs in the majority (91%) of biopsies from patients with CLE (including LET, CCLE, and SCLE) and in none of those from patients with polymorphous light eruption, and they concluded that the 2 conditions probably have a different etiology and pathogenesis.
Reticular erythematous mucinosis most often affects young women and typically presents as a reticulated macular or papular erythema. Biopsy of the lesions reveals a periadnexal and perivascular lymphocytic infiltrate associated with interstitial mucin deposits.48 Patients also usually report marked photosensitivity. Because of this, some authors have considered reticular erythematous mucinosis to be a variant of CCLE or LET,12 though it is important to note the finding of immunoglobulin M deposits on the basement membrane in some cases of reticular erythematous mucinosis.49 However, no comparative studies between the 2 conditions that would allow this theory to be confirmed have been published.
ConclusionsLET is a form of lupus erythematosus with a specific clinical presentation that differentiates it from the classic forms of CLE. Although the first descriptions of the histological features of LET highlighted an absence of the specific epidermal alterations characteristic of lupus, some cases do show such changes, though these are only ever mild or moderate. The occasional presence of epidermal alterations in LET should be taken into account in clinical practice so that cases of LET in which damaged epidermis is observed in the biopsy are not erroneously diagnosed as CCLE or SCLE.
While there is now published evidence to suggest that LET is a true form of lupus and not a separate disease, the position it should occupy in the classification of CLE continues to be an issue. However, in light of the clinical differences that distinguish LET from CCLE, it is reasonable to classify it as a separate subtype, which some authors have called intermittent cutaneous lupus erythematosus.
LET displays certain characteristics that differentiate it from polymorphous light eruption. There is, however, little to differentiate it from Jessner lymphocytic infiltration and reticular erythematous mucinosis, conditions that tend to be included within the spectrum of LET at the present time.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Carundo C, Bielsa I. Lupus eritematoso túmido, una entidad en proceso de definición. Actas Dermosifiliogr.2011;102:688-674.