The pine processionary caterpillar is the larval form of the Thaumetopoea pityocampa moth. Mediterranean forests regularly suffer plagues of this insect, which has been moving north as a result of global warming. When the small urticating hairs that develop during the last 3 larval stages are shed and can become airborne. If they come in contact with skin, they can cause a variety of reactions, notably contact urticaria and papular rashes. Irritation can also occur if the hairs lodge in the mucosa of the conjunctiva or in the respiratory tract. Several cases of anaphylactic reactions have been reported in recent years. Mechanical (irritative) mechanisms may be involved in the pathogenesis of lesions, or immunoglobulin E-mediated allergic hypersensitivity reactions may be implicated when the process is rapid, recurrent, and progressively more severe.
La oruga procesionaria del pino es la forma larvaria del lepidóptero nocturno Thaumetopoea pityocampa (TP). Supone una plaga forestal en los países mediterráneos y se está expandiendo hacia el norte de Europa por el calentamiento global. Durante sus tres últimos estadios larvarios presenta unos pelos urticantes de pequeño tamaño, que se desprenden con facilidad y pueden ser transportados por el viento. Estos pelos pueden producir distintas patologías, entre las que destaca la afectación cutánea que se manifiesta fundamentalmente como urticaria de contacto y dermatitis papulosa. También son capaces de clavarse e irritar la mucosa conjuntival y de penetrar en la vía respiratoria produciendo manifestaciones a este nivel. En los últimos años se han descrito varios casos de reacciones anafilácticas por este insecto.
Los mecanismos patogénicos implicados incluyen el mecánico o irritativo y el alérgico por hipersensibilidad mediada por IgE, donde las reacciones son inmediatas, repetidas y progresivamente más graves.
Lepidopteras are one of the most numerous orders of insects in the world, with almost 150 000 species described.1 Caterpillars—the larval form of these insects—are responsible for most of the adverse reactions in humans, although such reactions have also been reported after contact with the adult insect (moths or butterflies). This is because caterpillars, which are the target of many predators, have defense mechanisms such as sharp spines, small urticating hairs, and a range of toxic substances.2–4
The terminology used to describe reactions to lepidopteras is confusing and, at times, contradictory. The term erucism is derived from the Latin eruca, or caterpillar, and so should be reserved for any disease caused by caterpillars or the larval phase of these insects. The word lepidopterism is derived from the Greek lepis, or scale, and pteron, or wing, and its use should be limited to reactions caused exclusively by lepidopteras in the adult reproductive phase, ie, moths and butterflies. However, these terms are often used incorrectly, and erucism is used for exclusively cutaneous reaction to lepidopteras in any phase of development, and lepidoterism is used when there is noncutaneous involvement. In line with the approach of a recent review,2 we will not use this terminology but rather refer directly to the type of clinical manifestation and its cause (for example Thaumetopoea contact urticaria).
This article will focus on the skin diseases caused by the pine processionary caterpillar (Thaumetopoea pityocampa), one of the main tree pests in southern Europe and other Mediterranean regions,5–7 and the most common cause of adverse reactions to lepidopteras in Spain. Recent studies also point to the expansion northwards of this insect as a result of global warming, and a greater incidence of disease in humans due to the growth of residential areas on the outskirts of towns, many of which are close to pine forests infected by pine processionary caterpillars.5,8,9T pityocampa feeds on the needles of different conifer species, including the stone pine (Pinus pinea), from which pine nuts are obtained for human consumption. Spain is the leading producer of these in the world (Fig. 1).
In the larval phase, the caterpillars are full of microscopic urticating hairs able to penetrate the epidermis and mucous membranes. The most frequent clinical manifestations are cutaneous, although cases of eye involvement, rhinitis, and even respiratory conditions and anaphylaxis have been reported. Explanations for these reactions include mechanical or irritant mechanisms, chemical or toxic mechanisms after substance release (currently out of favor), and triggering of an allergic immunoglobulin (Ig) E-mediated reaction to different caterpillar proteins.2,3,10–21
Greater awareness among dermatologists of the biology of T pityocampa and the reactions it causes will enable correct diagnosis and appropriate treatment.
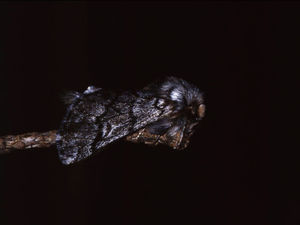
BiologyThe pine processionary moth (T pityocampa) is a nocturnal lepidoptera of the Thaumetopoeidae (Notodontidae) family (Fig. 2). It is found in parts of Europe, northern Africa, and the Middle East. In Europe, there are 3 species of Thaumetopoea: Thaumetopoea pinivora predominates in the north, Thaumetopoea processionea in central regions, and T pityocampa in Mediterranean regions.22 In Spain, it is present throughout the peninsula and the Balearic Islands, although it is mainly found in central and southern parts. It can affect all pine species, whether autochthonous or not (Pinus pinaster, Pinus silvestris, Pinus halapensis, Pinus nigra, Pinus pinea, Pinus radiata, Pinus ponderosa, and Pinus canariensis) and also cedar species.7
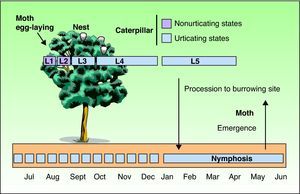
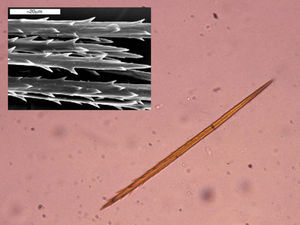
As with all lepidopteras, T pityocampa goes through 4 phases of development: moth, egg, larva, and chrysalis. Its biological cycle (Fig. 3) is heavily influenced by climate. For this reason, its spread northwards is being studied as a biological marker in the context of climate change. Moths represent the adult phase of the reproductive cycle. After fertilization, the eggs are deposited in the needles of pines and hatch after 30 to 40 days. The caterpillars themselves go through 5 larval stages. From the start, they are social and have a characteristic way of moving in single file, as if in a procession, hence their common name. From the third larval stage (L3), which takes place between September and November according to the climate, the colony forms readily visible “tents” or nests in the tops of the pine trees (Fig. 4). Although the caterpillars have large hairs visible to the naked eye right from birth, from stage L3 onwards, microscopic hairs also appear in the 8 orange-colored tori along their back. These microscopic elements, similar to small hairs (known in the scientific literature as setae), are between 150 and 200μm long and 5μm in diameter. Unlike true hairs, they are not innervated and readily detach from the skin with the slightest mechanical shock or stimulus.10 From L3 onwards, with each shedding, new hairs are produced while the old ones remain in the larval remnants. Likewise, the cocoons in which pupation occurs underground are also coated with urticating hairs.10 These act as harpoons and are able to lodge in the skin or the ocular and respiratory mucosa (Fig. 5). In addition, their presence in air has been shown by techniques used to detect pollen or airborne microorganisms.23 They can therefore cause problems in the absence of direct contact with the caterpillars. The abundance of these hairs in the air is related to the distance from the areas where the hairs are produced, weather conditions (larger quantities will travel further on windy days), and stage in the biological cycle (progressive increase from stage L3, with each caterpillar having more than 1 million hairs in its fifth and final stage, L5).24 The hairs can attach to objects (wood, pine cones, clothes, etc) or the hides of animals (pets or livestock) and cause symptoms outside pine forests. They can also persist for a long time, even years, in the environment, and so patients may show reactions throughout the year.13,25 In the first days of spring (between January and May depending on the region), mature caterpillars (L5) leave the pine trees in a procession, led by a female to a place where they burrow into the ground (edges of the forests or clearings in mountainous areas in cold or temperate habitats and shady areas in warmer areas) (Figs. 6 and 7). It is therefore at this time, during the L5 stage, when the greatest number of reactions to the insect occur as the chances of contact are higher and the number of hairs and allergenicity of the caterpillars are greatest.26 Underground, the caterpillars move into the chrysalis phase (Fig. 8) in which there is a period of diapause or break in development. The duration of this phase is very variable (from under a month to up to 4 years, according to weather conditions).7 This makes it difficult to control plagues. Hairs and caterpillar remains are also present in the ground all year round, with the resulting clinical repercussions for visitors and workers who disturb the soil of a pine forest. Finally, the moths emerge from the ground on summer days to initiate a new cycle.
Group of caterpillars burying themselves in the ground. Note that they have large hairs (2-3mm long) and other much more numerous small ones (0.2-1mm) located on the back. These give the characteristic brown color of the pine processionary moth and are responsible for the reactions caused.
Currently, the importance of disease caused by T pityocampa is underestimated in both clinical practice and the scientific literature, probably because most reactions are mild and transient, and often the patient is aware of the cause and does not consider it important or self-treats the condition. However, sometimes, clinical suspicion is not so clear and there are many possible diagnoses. Moreover, we should also bear in mind that more serious reactions may occur and these are treated symptomatically without a diagnosis of the cause or without providing the patient with the necessary information to avoid future recurrences.
Another factor to take into account is that, although the reactions usually occur in isolated patients, at times, epidemics may occur that are related to exposure in areas of heavily infested pine forest, the biological cycle of the caterpillar (February to April), or weather conditions, particularly strong winds that readily disperse the urticating hairs. In addition, T pityocampa may remain in chrysalis form for several years if weather conditions are unfavorable, and several generations of caterpillar may emerge at the same time, with greater risk of an epidemic of disease.25
Few epidemiological data have been published on reactions caused by T pityocampa. Recently, a cross-sectional, randomized, age-, sex- and habitat-adjusted study of 1224 participants was conducted in the province of Valladolid, Spain (an endemic area with large areas of pine forest destroyed by T pityocampa).9 The prevalence of skin reactions to T pityocampa was 12% in rural areas, 9.6% in semiurban areas with nearby pine forest, and 4.4% in urban areas. It was found that the risk of skin reactions to T pityocampa was directly related to exposure to the caterpillar, and not to age or atopic predisposition. Workers in forested areas are therefore the group most at risk of T pityocampa–related disease (the risk is 5 times higher). In addition, many of these workers have IgE-mediated sensitivity and so are at risk of more severe reactions. The occupations at greatest risk are pine cone collectors and lumberjacks, followed at some distance by resin collectors, farmers, forest wardens, gardeners, bricklayers, and haulage contractors who work in regions of pine forest.9,12–14,17 It should be remembered that symptoms may occur at other times, coinciding with the start of the pruning season and pine cone collection in the fall, or wood and sand collection in summer months.
Children are particularly susceptible to lepidoptera-related conditions, more than likely because their natural curiosity leads them to touch the caterpillars or play with sand or vegetation that contains these insects.15,19,27 One study of the prevalence of skin reactions to T pityocampa in children in a rural endemic area showed that 9.2% of children had a history of some sort of reaction.15 This prevalence may increase in peripheral urban or semiurban areas with infested pine forests nearby or on the land where children play if pine trees are present.
Pathogenic MechanismsThe irritant nature of Thaumetopoea larvae has been known since the times of ancient Greece,10 and some sources speak of the Romans throwing prisoners in pits full of pine processionary caterpillars as a severe form of punishment.28 However, the first descriptions of the pine processionary caterpillar were made by Reamur in 1736 and subsequently by Fabre in 1900.29 Since then, and for years, it has been accepted that the reactions to T pityocampa resulted from mechanical irritation arising when the hairs of the caterpillar lodge in the skin and the release of toxic or irritant substances.
The toxic mechanism has been demonstrated in caterpillars of different lepidopteras. Penetration of the skin by urticating hairs leads to basophil degranulation, with the subsequent histamine release. In the case of T pityocampa, this IgE-independent degranulation has been associated with a protein known as thaumetopoein present in the hairs of the caterpillar.30 However, this mechanism has been challenged recently in a study that demonstrated the slow onset of skin lesions after intracutaneous exposure to hairs of another irritating species of Thaumetopoea (T pinivora), thereby casting doubt on the part played by fast mediators such as histamine.22 This, along with the fact that pretreating the hairs with different chemical products or heating them does not alter their inflammatory action, rules out, according to some authors, the participation of a toxic mechanism in Thaumetopoea-related disease.10
The fact that some individuals present with more intense, immediate reactions while others show minimal or even no clinical manifestations after similar exposure has led some authors to suspect that an IgE-mediated allergic mechanism may be present. In 1993, Werno et al21 found IgE antibodies against thaumetopoein (28 kDa) in 20% of a group of exposed workers. Moneo et al31 purified a 15-kDa protein (Tha p1) which was recognized by IgE antibodies of patients allergic to T pityocampa. This mechanism has now been demonstrated in several studies by immunoblotting of specific IgE as well as positive skin prick tests performed with total larval extract at nonirritant concentrations (previously tested in controls).12–20
In endemic areas, skin prick testing with caterpillar whole body extract has yielded positive results in 53% to 58% of individuals with suspected T pityocampa reactions.13 Although this technique is very sensitive, specificity is low. However, the results of IgE immunoblotting were positive in 72% of the patients with positive skin prick tests, thereby demonstrating the usefulness of these tests.13 In view of these results, it seems that IgE-mediated hypersensitivity allergic reactions to this insect are at least as important as those with no allergic mechanism.
To date, there has been no conclusive evidence of the participation of other types of hypersensitivity mechanisms, although cellular immunity is probably involved in some cases. It also seems that chitin and its degradation products, which are powerful promoters and regulators of immune reactions, may play a role in the variable sensitivity to this insect.10
In summary, mechanical irritation of the skin is responsible for the reaction after contact with T pityocampa in all exposed individuals, and in some susceptible individuals, a more severe IgE-mediated allergic reaction may be triggered. What remains to be demonstrated is a toxic mechanism arising from release of chemical substances and the participation of other immune pathways in reactions to T pityocampa.
Pathologic AnatomyAlthough the histopathologic findings in patients after contact with lepidopteras are not specific, certain patterns, which may vary from one species to the next, have been reported. Some of these include localized necrosis, blister formation, foreign body granulomatous reactions, predominantly lymphocytic inflammatory infiltrates, presence of eosinophils, etc.32
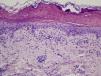
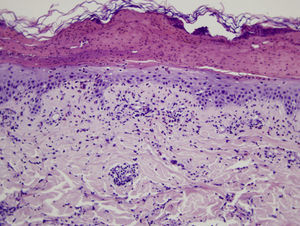
Microscopic assessment of the different skin reactions to T pityocampa is also nonspecific and cannot be used for diagnosis. Different types of skin reaction to T pityocampa have been described, namely contact urticaria and different types of dermatitis (papular, vesicular, and pustular16,33). The vesiculopustular type is most frequent in children and there have been no histopathological descriptions. The microscopic pattern of the most characteristic cutaneous manifestation, papular dermatitis, has been described. A nonspecific inflammatory reaction is observed with epidermal edema and a perivascular lymphohistocytic infiltrate with eosinophils, as seen in bites and reactions to other insects (Fig. 9).
Clinical ManifestationsSkin involvement is the most common clinical manifestation after exposure to T pityocampa. Ocular manifestations are also frequent and there have been reports of respiratory symptoms and even anaphylactic reactions, although these are becoming rarer.12–20,33
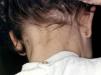
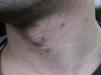
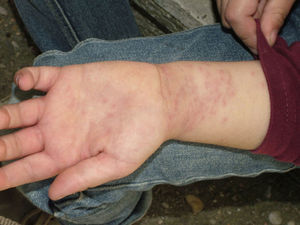
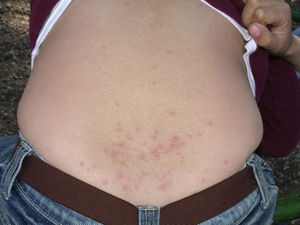
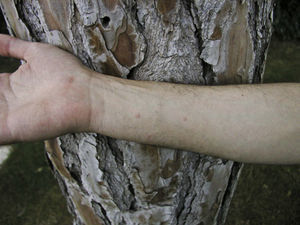
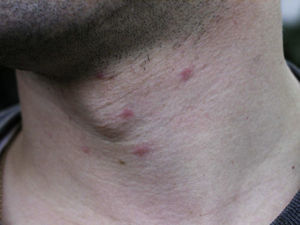
Skin lesions are more frequently located on exposed areas, typically on the neck and limbs, and in particular the wrists, forearms, and ankles, although covered areas of the body may also be affected. The palms of the hands and the interdigital spaces are more often affected in children, probably due to direct contact with caterpillars when playing on infested land.15 Physical activity and scratching may increase the intensity of the dermatosis.
Skin reaction to T pityocampa may take on several patterns. The most frequent ones are papular dermatitis and contact urticaria. Vesiculopustular rash and infiltrated papules similar to insect bites have been reported. These may last days.
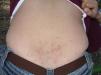
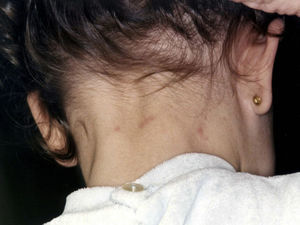
Papular dermatitis is characterized by the appearance of a papular, erythematous rash, with severe pruritus, numerous lesions caused by scratching, and eczematous areas (Figs. 10–13). These lesions usually appear within hours of contact and they persist for several days.
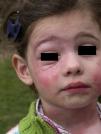
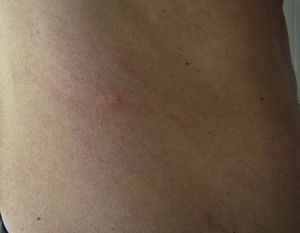
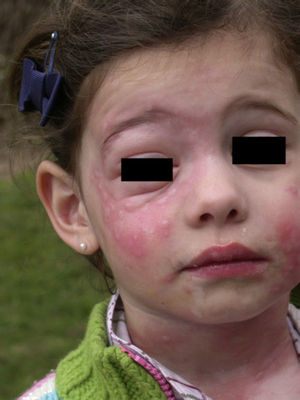
In the clinical course of contact urticaria, evanescent itchy bumps (lasting from minutes to a few hours) form, often in association with angioedema, particularly on the eyelids (Fig. 14).14 Urticarial lesions sometimes show certain infiltration, resembling papular urticaria, and so they may persist for several days (Fig. 15).
Less frequently, papulovesicular and even pustular lesions have been reported, mainly on the palms of the hands of small children.16 In general, the lesions are accompanied by intense itching and tend to disappear in a few days.
The characteristic ocular symptom is conjunctivitis, which is aggravated by scratching as this increases the penetration and friction of urticating hairs. Less frequently, cases of keratitis and opthalmia nodosa have been reported. Respiratory involvement is less common, and manifests mainly as dyspnea, occasionally associated with an anaphylactic reaction with multiorgan involvement in several cases reports.12–15,17,20
In nonallergic patients, the most common manifestation is pruritic papular dermatitis (described earlier), although contact urticaria, generally more localized, may also present. Less frequently, T pityocampa causes a delayed skin reaction that can last days and that is manifested in the form of small infiltrated papules, papulovesicles, or pustules (Fig. 16).
The clinical pattern of patients allergic to T pityocampa (with positive skin prick tests/serology tests and with reaction after exposure) shows significant differences with respect to nonsensitized patients. These are patients who experience reactions, even after minimal exposure (walking through a pine forest without any evident contact with the caterpillars), with immediate onset of symptoms although these are shorter lasting and progressively more intense (more cases of generalized urticaria, angioedema, and anaphylaxis).12–20
In allergic patients, the characteristic skin manifestation is contact urticaria accompanied by angioedema in half the cases. As with other contact allergy urticarias, after contact, which can be minimal, the lesions appear quickly, usually during the first hour, and often spread to covered areas of the body and may even become generalized. Respiratory symptoms occur more frequently in individuals allergic to T pityocampa, and cases of anaphylactic reactions have been reported in such individuals. These reactions are more frequent and more severe in workers in close and repeated contact with T pityocampa.
DiagnosisThere are no specific clinical signs of reactions to T pityocampa. Diagnostic suspicion of a cutaneous reaction to T pityocampa is based on the following observations:
- 1.
History of exposure in the previous 24hours in an area with pine trees infested with T pityocampa in any period of the year, although symptoms are more frequent between February and April. In workers in contact with the pines, exposure will depend on the type of work that they do. Pine cone collectors have a peak in the incidence of reactions between October and December. Reactions have also been observed in the middle of summer in workers who remove sand from pine forests in this period, when there are no irritating caterpillars although remains or chrysalises may be present in the sand.
- 2.
Presence of itchy bumps, with or without angioedema or papular dermatitis, that are very pruritic and tend to appear on the neck and distal areas of the limbs. In children, the palms and interdigital spaces in particular should be checked for lesions.
- 3.
Identification, whenever possible, of urticating hairs on the skin or clothes of the patient by applying an adhesive strip (cellophane or surgical tape) or directly through use of a dermatoscope. This technique has been used recently in the diagnosis of different skin infestations (entodermoscopy) and can be considered as a useful tool in the differential diagnosis of papular or erythematous rashes.34,35
- 4.
Reactions should not appear in other circumstances, and other differential diagnoses that may be associated with similar signs and symptoms should be excluded. These include reaction to bites of other insects, nodular or atopic prurigo, scabiosis, other contact eczemas and urticarias, etc.
In the previous section, we commented on the clinical criteria that suggest participation of an IgE-mediated allergic mechanism. Confirmation of such a mechanism would be made by a positive result in the skin prick tests and/or specific IgE determination in serum for a caterpillar extract (Table 1).
Differences Between Individuals With Allergic and Nonallergic Reactions to Thaumetopoea pityocampa.
| Allergic | Nonallergic | |
| Prior contacts with the caterpillar (prior immunization phase) | Necessary | Not necessary |
| Characteristic skin manifestation | Contact urticaria | Papular dermatitis |
| Latency period | <1 hr | >1 hr |
| Severity of the clinical manifestations | Greater | Lower except for extensive exposure |
| Skin prick test | Positive | Negative |
| Specific IgE | Yes | No |
Abbreviation: IgE, immunoglobulin E.
In certain areas, T pityocampa could be described as a plague, with infestation of many different trees and even entire pine forests. Different techniques are being used in an attempt to control this pest. These include mechanical measures (placing adhesive bands impregnated with insecticide around the trees or directly destroying nests), fumigating with different chemicals or biological products, use of pheromones, and encouraging natural predators.7,36
Table 2 shows some of the preventive measures to avoid reactions to this insect. Once symptoms have appeared, treatment is exclusively symptomatic: oral antihistamines to control pruritus, contact urticaria, and angioedema, and topical corticosteroids for eczematous lesions and papular dermatitis. In patients with extensive or refractory lesions, oral corticosteroids can be used. Scratching should be avoided as far as possible as this will exacerbate symptoms when the caterpillar hairs rub against or become lodged in the skin or mucosas. In the event of anaphylactic reactions, early diagnosis is needed, along with immediate treatment with epinephrine as well as corticosteroids and antihistamine agents.
Recommendations for Avoiding Reactions to Processionary Pine Caterpillar, of Particular Importance for Patients Allergic to the Insect.
| In the months in which the caterpillar descends in processions from the pines (February to April in cold winters, January to March in milder winters), it is recommended not to walk through pine forests infested by the insect, especially on windy days. Pets (for example dogs) should also avoid these forests at this time. |
| If caterpillar processions are seen, keep children away and NEVER disturb, touch, or sweep them (thousands of irritating darts will be released). If they are on your land, once they have gone underground, you can moisten the surrounding area to ensure that the hairs of the caterpillars remain on the ground. |
| Do not collect objects (pine cones, wood, etc) from infested pine forests or touch the caterpillar nests. If you live on land with pines, in the last months of winter and in spring, do not leave your washing outside to dry. |
| Avoid disturbing the earth in pine forests or the surrounding area. |
| In cases of occupational exposure, precautionary measures are essential when working in infested pine forests. It is important to leave as small an area of skin exposed as possible and to wear appropriate clothing (eg, shirts and trousers that cover your arms and legs) and footwear (eg, boots). If you are going to perform tasks at the tops of pine trees, use protective goggles or even a mask. Patients allergic to the caterpillar should not work in infested pine forests. |
The pine processionary moth is a pest that is spreading, particularly in Mediterranean countries. In addition to the deforestation it causes, with the ensuing environmental and economic impact, the larval form of this moth is responsible for clinical reactions in humans, which are often underdiagnosed by dermatologists.
The urticating hairs of this caterpillar can induce a range of skin, conjunctival, and respiratory reactions, and even severe anaphylactic reactions. Although considered a seasonal affliction mainly observed between February and April, T pityocampa is responsible for symptoms throughout the year. History of exposure to the caterpillar in visitors and residents of areas of pine forest and, above all, in pine industry workers is an essential part of the diagnosis. Criteria for suspicion of an IgE-mediated allergic component include immediate and repeated reactions that become progressively more severe, even with minimal exposure.
We believe that dermatologists should become familiar with disease caused by T pityocampa, as skin manifestations are without doubt the most common and often the only clinical symptom. Correct diagnosis and appropriate information that emphasizes preventive measures will reduce the incidence and severity of these reactions.
FundingThis study was funded in part by the URTICLIM project of the French National Research Agency.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as. Vega J, et al. Manifestaciones cutáneas por la oruga procesionaria del pino (Thaumetopoeapityocampa). Actas Dermosifiliogr.2011;102:658-667.