Leprosy is a chronic granulomatous disease caused by the bacillus Mycobacterium leprae. It primarily affects the skin and peripheral nerves and is still endemic in various regions of the world. Clinical presentation depends on the patient's immune status at the time of infection and during the course of the disease. Leprosy is associated with disability and marginalization.
Diagnosis is clinical and is made when the patient has at least 1 of the following cardinal signs specified by the World Health Organization: hypopigmented or erythematous macules with sensory loss; thickened peripheral nerves; or positive acid-fast skin smear or skin biopsy with loss of adnexa at affected sites.
Leprosy is treated with a multidrug combination of rifampicin, clofazimine, and dapsone. Two main regimens are used depending on whether the patient has paucibacillary or multibacillary disease.
La lepra es una enfermedad granulomatosa crónica causado por una micobacteria (M. leprae) que presenta predisposición por la piel y nervios periféricos. La lepra continúa siendo endémica en distintas regiones del mundo. La presentación clínica de la enfermedad depende del estado inmunológico del paciente al adquirirla y durante la evolución de la misma. Es una infección que se asocia a discapacidad y marginación.
El diagnóstico de lepra es clínico y se hace al tener uno o más de los signos cardinales establecidos por la OMS; máculas hipopigmentadas o eritematosas con disminución de la sensibilidad, engrosamiento de los nervios periféricos y la demostración de bacilos ácido alcohol resistente en una baciloscopia o biopsia de piel, con pérdida de anexos en los sitios afectados.
El tratamiento es con tres drogas; rifampicina, clofazimina y dapsona. Existen principalmente dos modalidades de tratamiento dependiendo de la presentación clínica del paciente como paucibacilar o multibacilar.
Hansen disease, or leprosy, is a chronic granulomatous bacterial infection that primarily affects the skin and peripheral nerves. The disease is caused by an obligate intracellular bacillus, Mycobacterium leprae, which was identified in the 19th century by the Norwegian physician Gerhard Henrik Armauer Hansen.1 The clinical presentation and histopathologic changes depend on the immune status of the patient at the time of infection and over the natural course of the disease. Diagnosis is currently based on 3 cardinal signs specified by the World Health Organization (WHO): hypopigmented or erythematous macules with sensory loss, thickened peripheral nerves, and a positive acid-alcohol-fast smear or skin biopsy.2 Modern multidrug therapy and new antibiotics of proven efficacy have made it possible to meet the WHO's targeted reduction in the incidence of Mleprae infection to a single case per 10000 inhabitants in countries where the disease is endemic. A new pathogen, Mycobacterium lepromatosis, has recently been found to cause endemic disease in Mexico and the Caribbean.3 These developments call for new medical perspectives on how to cope with a problem that is still far from resolved.
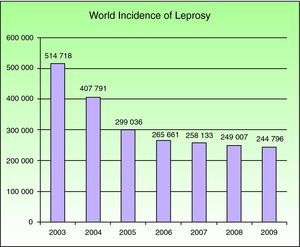
EpidemiologyNational leprosy programs implemented from 2006 through 2010 were successful in meeting the WHO's target for regions where leprosy is endemic. Reports on the situations in 141 countries were sent to the WHO in the final months of 2010. A total of 244 796 new cases were registered in 2009, with southeast Asia having the largest number (166 115 new cases); at the beginning of 2010 the worldwide prevalence was 211 903 cases.4 Currently this mycobacterial infection is endemic in more than 15 countries, but 83% of the cases are found in 3 countries: India, Brasil, and Birmania.2,4 India registered 64% of all cases. The reported prevalence of leprosy was 212 802 cases in 2008, and 2007 saw 254 252 new cases registered. The number of cases fell by 11 100 (4%) from 2006 to 2007 (Fig. 1).
Number of new cases registered from 2003 to 2009 in 16 countries reporting more than 1000 cases each year. Source: Global Leprosy Situation 2010.4
At the beginning of the 1990s, the WHO proposed their “final push strategy” for leprosy with the clear purpose of elimination, defined as a prevalence below a single case per 10 000 inhabitants in endemic regions.4 Countries like the Democratic Republic of the Congo and Mozambique reported reaching the goal, but the disease remains highly prevalent in other parts of the world. Lower prevalence rates are unrelated to the reduction in the number of new cases found. This change in prevalence does not reflect a decrease in M leprae transmission; rather, it is related to the shorter period of treatment recommended by the WHO or to the exclusion from registries of patients who have been cured or who have died.
Countries where leprosy had previously been eliminated report a rise in imported cases; one example is Spain, where most imported cases have come from South America or sub-Saharan Africa.5
Microbiology and ImmunologyMleprae is an acid-alcohol-fast, gram-positive obligate intracellular bacillus that shows tropism for cells of the reticuloendothelial system and peripheral nervous system (notably Schwann cells); this mycobacterium is the only one with these characteristics. The taxonomic order is Actinomycetales, the family Mycobacteriaceae. Mleprae organisms are slightly curved, measure from 1 to 8μm in length and 0.3μm in diameter; like other mycobacteria, they replicate by binary fission.
The leprosy bacillus has a predilection for macrophages, collecting in intracellular groups called globi. Although never cultured in vitro, Mleprae has been grown in the foot pads of 9-banded armadillos. Replication takes from 11 to 13 days, considerably longer than the 20hours required by Mycobacterium tuberculosis. Predisposed to infect cold areas of the body such as the skin, nasal mucosa and peripheral nerves (especially superficial ones), Mleprae grows best at temperatures between 27°C and 30°C. The efficacy of this pathogen within a narrow ecological niche is primarily explained by the properties conferred by 2 structural elements: the capsule and the cell wall.6
The capsule is made up of a large number of lipids, mainly phthiocerol dimycocerosate and phenolic glucolipid-1, which is the target of an intense immunoglobulin M-mediated humoral immune response.2,6,7 Another important component of the cell wall is lipoarabinomannan, which is an antigen for the macrophage. Many of the functional genes found in other mycobacteria have been silenced or transformed into pseudogenes, thereby inactivating functions such as extracellular reproduction. Thus, a set of metabolic and reproductive functions make M leprae an obligate intracellular bacterium with a long reproductive cycle.
M leprae has a predilection for Schwann cells, explained by specific binding to the G domain of the laminin-α2 chain, which is expressed specifically in the basal lamina of peripheral nerves. Once the pathogen penetrates a cell, replication proceeds slowly until T cells recognize the mycobacterial antigens and a chronic inflammatory reaction begins.7
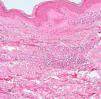
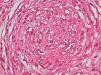
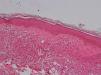
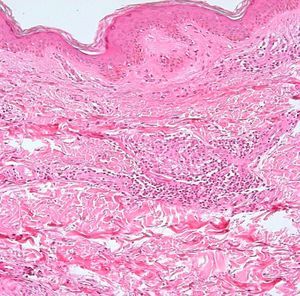
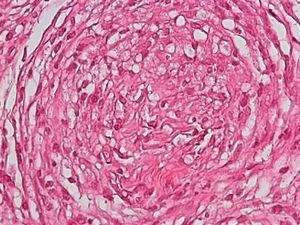
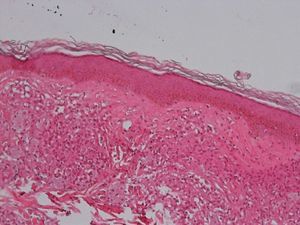
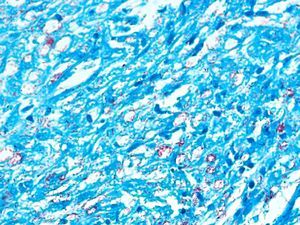
Development of the disease, with clinical manifestations, depends on the patient's immune status. A role for genetics, associated with a susceptibility locus at chromosome 10p13 near the mannose receptor 1 gene, has now been suggested; mannose receptors on the surface of macrophages are important in phagocytosis.8 In addition, class II HLA/major histocompatibility complex genes at chromosome 6 have also been implicated in the type of leprosy a patient develops. The HLA-DR2 and HLA-DR3 genes have been linked to tuberculoid leprosy whereas HLA-DQ1 is most often found in patients with the lepromatous form. Many other immune system components have been associated with clinical phenotype and course of disease. An intense, organized, specific cellular response is seen in cases at the tuberculoid pole, whereas an absence of a specific immune response is seen at the opposite pole, in lepromatous leprosy. The lepromatous form affects the skin and peripheral nerves, causing well-defined infiltrated plaques that are annular or ovoid. These lesions are usually anesthetic and may affect any area of the body. Biopsy of the skin and region surrounding nerves reveals granulomas with an abundance of epithelioid histiocytes, multinucleated giant cells, and CD4+ T cells that secrete interferon-γ. One of the most important findings is the scarcity or absence of acid-alcohol-fast bacilli (Figs. 2 and 3), although a few may sometimes be observed. The immunologic and clinical situation is different in lepromatous leprosy as there is no specific immune response. Bacilli proliferate in the tissues and foamy macrophages can be observed; few CD4+ and CD8+ T cells are present, and granulomas do not usually form (Figs. 4 and 5). Immunohistochemical findings in skin biopsies show mainly interleukins 4 and 10.2
The immune response to Mleprae is variable and gives rise to spontaneously changing clinical manifestations, which may present as type 1 or 2 leprosy reactions. Reactions are related to immune system changes, such as those caused by antileprosy medication, stress, or pregnancy. A type 1 reversal reaction involves type IV hypersensitivity.9 Blood levels of cytokines—such as interferon-γ and tumor necrosis factor—rise and CD4+ T cells are activated. A type 2 (or erythema nodosum leprosum) reaction corresponds to a type III hypersensitivity reaction due to immune complex deposition associated with systemic toxicity, elevated tumor necrosis factor levels, and increased neutrophilic infiltration and complement deposition in the skin. This type develops mainly in cases of dimorphous (borderline) leprosy and lepromatous leprosy.
ContagionAlthough the mechanism by which the pathogen is transmitted is poorly understood, we do know that the transmissibility of M leprae is low. Overcrowding and prolonged contact are known risk factors.10 The possibility that the respiratory tract plays a significant role in transmission has recently been studied. The bacterial load is high in patients with lepromatous leprosy, who have been reported to harbor as many as 7000 million bacilli in a gram of tissue, whereas in other forms of the disease, the load is known to be much lower, on the order of a million bacilli in total. M leprae has been found in high numbers (100 million viable bacilli per day) in nasal mucosa.11 Although the skin has been suggested to be a possible route of transmission, this hypothesis has never been proven. As the viability of bacilli outside the body extends over a period ranging from 36hours to 9 days, fomites can play a role in transmission. The respiratory tract may provide a point of entry for the bacillus, and aerosol inoculation has been shown to be possible in immunodeficient mice.
No relationship between the bacillus and a vector has been established, but the possibility cannot be ruled out. As leprosy is not a highly contagious disease, certain conditions must be met before a host can be infected. Cases of tuberculoid leprosy transmitted through tattooing have been reported, mainly in India.12 As vertical transmission has been reported, mother-child dyads should be followed.13
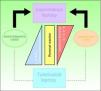
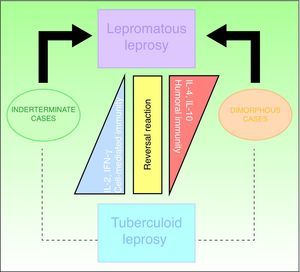
ClassificationThe Ridley-Jopling clinical classification system (Fig. 6), based on the patient's clinical state and immune status, is the most widely used.14 The disease is organized in 2 poles and an intermediate state, referring to lepromatous, tuberculoid and dimorphous (borderline) leprosy, respectively. Dimorphous cases are classified according to which pole (lepromatous or tuberculoid) they tend toward, preceded by the word borderline (thus, cases are borderline lepromatous, borderline tuberculoid, or borderline borderline). Indeterminate cases are considered to represent the initial stage of the disease. These unstable cases eventually move toward one of the poles but progression can be halted with treatment and a cure is possible. In fact, a cure is easy in this stage, although clinical diagnosis is difficult. All dimorphous or indeterminate cases progress toward a pole, usually becoming lepromatous. In 1998, the WHO's Expert Committee on Leprosy determined that treatment could be started before smear tests were done; thus, a practical, rapid means of classification was established for worldwide application without need for diagnostic equipment and without putting health care workers at risk.2,4,10 Paucibacillary cases are those in which skin lesions number no more than 5; cases with 6 or more skin lesions are classified as multibacillary.15 This system is imperfect, however, as a large number of multibacillary cases are misclassified as paucibacillary, with repercussions on treatment.
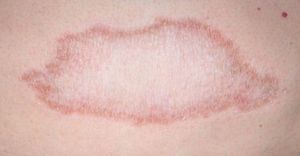
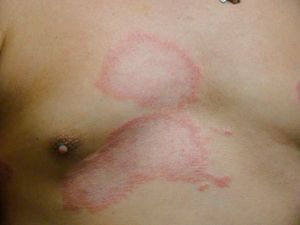
Clinical FindingsLeprosy affects primarily the skin, superficial peripheral nerves, the eyes, and certain organs (e.g., the testicles). A disseminated skin condition is often the reason patients seek care, although they may also complain of numbness and other types of paresthesia or systemic signs such as fever and weight loss. Lepromatous leprosy (Fig. 7) is considered to be at the dynamic, progressive, systemic, and infectious end of the spectrum. Bacteriology will be positive and the Mitsuda reaction (intradermal lepromin test) will be negative due to the absence of specific cell-mediated immunity. Tuberculoid leprosy (Fig. 8) is stable, rarely contagious, and may even be self-limiting. The bacillus is not detectable on bacteriology, but the Mitsuda reaction will be positive and granulomas are typically found on biopsy.
Tuberculoid LeprosyAt the tuberculoid pole (tuberculoid and borderline tuberculoid cases) the disease manifests with a few well-defined, hypopigmented anesthetic macules. Lesion borders are elevated and erythematous and the centers are atrophic. There is usually no loss of sensation on the face because of the abundant sensory innervation there. This form is associated with anhydrosis and loss of adnexal structures. Because the patient is immunocompetent, lesions are not usually large or numerous, and this type of leprosy may resolve spontaneously if the host's immune system is strong.
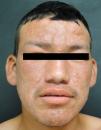
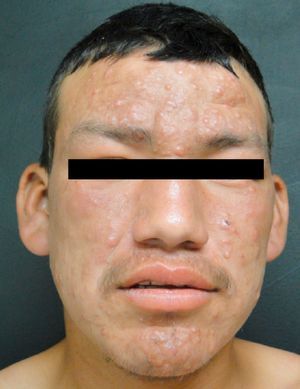
Lepromatous LeprosyThe lepromatous pole of the spectrum (lepromatous leprosy and borderline lepromatous cases) is characterized by confluent papules and nodules, possibly resulting in marked, diffuse infiltration of the skin and giving rise to leonine facies and madarosis. Lesions are usually symmetrical and bilateral. Early in the disease the skin appears infiltrated and waxy. This pole of the leprosy spectrum is characterized by greater nerve involvement and more severe disability. Nodular and diffuse forms of lepromatous disease have been observed.
Dimorphous CasesThe clinical presentation of dimorphous leprosy (Fig. 9) may be acute or subacute and cases are initially indeterminate. Considered an unstable, transient clinical state, dimorphous leprosy requires appropriate treatment. Nearly all cases progress to lepromatous disease. Patients with dimorphous leprosy present with scaly, erythematous plaques that may be circular or annular, with borders that are diffuse externally and well defined internally. With time the lesion atrophies and there is loss of adnexa. On occasion, it has been suggested that when the external borders of annular lesions are well defined, the disease progresses to tuberculoid leprosy, whereas well-defined internal borders predict progression to lepromatous disease.
Acute ReactionsErythema nodosum leprosum (type 2 reaction) is accompanied by systemic symptoms with changes in the patient's general state of health: fatigue, weakness, fever, joint pain, and weight loss. This leprosy reaction develops in around 60% of patients with lepromatous leprosy and may recur several times along the course of disease.15 Painful nodules appear, mainly on the lower limbs but occasionally on the trunk. The course of these nodules is subacute. A variant of a type 2 leprosy reaction causes necrotic erythema, or Lucio's phenomenon, which consists of red congestive macules that progress to blisters and necrotic slough, followed by atrophic scarring. Immune complex deposition is the mechanism of action. The relationship between Lucio's phenomenon and M lepromatosis is under study.16
A reversal reaction (type 1) can develop in interpolar cases and is associated with hormonal changes, such as occur in the puerperium,17 or with drug treatments, particularly antileprosy regimens. This antigenic reaction is caused by variations in the patient's immune status and is due to a cell-mediated hypersensitivity mechanism that develops within months of starting treatment or after treatment has stopped. Typical manifestations are erythematous macules with a congestive appearance and blisters, ulceration, and/or necrosis. An important aspect to watch for in these patients is neuritis. Timely start of effective treatment, before irreversible damage has occurred, is essential.
Clinical Signs and SymptomsThe leprosy bacillus targets the peripheral nervous system, leading to the wide variety of clinical manifestations that characterize this mycobacterial infection.18 Lesions may affect cutaneous peripheral nerves, primarily the posterior tibial, cubital, medial and lateral peroneal nerves.19 A superficial perineural osteofibrotic reaction develops, making the nerves palpable during physical examination. Nerve involvement causes thickening, pain, and sensory and motor impairment. When small cutaneous nerve fibers become involved, the result is numbness, anhydrosis, and thermal sensory impairment. In pure neuritic leprosy the neuropathy is asymmetrical. This variety is most often seen in India and Nepal. Differential diagnosis must take into consideration that peripheral nerve thickening also occurs in other diseases, namely primary amyloidosis and other hereditary diseases (e.g., Charcot-Marie-Tooth, Dejerine-Sottas, and Refsum diseases).20
The musculoskeletal system is affected in 95% of cases.21,22 The most common skeletal signs are nonspecific, as sensory loss secondary to nerve damage leads to ulcers, deformities, and fractures. It is important to remember that osteoporosis is the second most common sign in patients with leprosy.23
Patients with the lepromatous form have been reported to develop testicular compromise, mainly atrophy and acute orchitis related to erythema nodosum. The eye may become involved, due to direct infiltration or through damage to the optic nerve. Eleven percent of patients with multibacillary disease have been reported to present with loss of vision at the time of diagnosis.19 A frequent variety in Mexico, described by Lucio and Alvarado in 1851, is diffuse lepromatous leprosy, which is characterized by diffuse infiltration that gives a myxedematous and atrophic appearance to the skin, with singular projections on the ears.24 The main ocular manifestations of leprosy are lagophthalmos, keratitis, and entropion.
DiagnosisLeprosy is diagnosed clinically on the basis of 3 cardinal signs set out by the WHO's Expert Committee on Leprosy in 1997.2,15 The diagnosis is made when an individual who has not completed a course of treatment has 1 or more of the following signs:
- 1.
A hypopigmented (or erythematous) anesthetic skin lesion
- 2.
A thickened peripheral nerve
- 3.
A positive skin smear or bacilli observed in a biopsy
When all 3 signs are present diagnostic sensitivity has been reported to be as high as 97% (Table 1).15 Although 90% of paucibacillary cases are diagnosed based on lesion count, up to 30% of multibacillary patients are underdiagnosed.
The WHO's Cardinal Signs for the Clinical Diagnosis, Classification, and Treatment of Leprosy.
| Cardinal Signsa | Classification for Treatmenta |
| Hypopigmented or slightly erythematous macules with evident sensory loss | Paucibacillary (1 to 5 skin lesions) |
| Thickened peripheral nerves | Multibacillary (6 or more skin lesions) |
| Positive acid-alcohol-fast smear or skin biopsy |
Any single cardinal sign is diagnostic and indicates the clinical classification for guiding treatment according to the World Health Organization (WHO). Source: Britton et al.2
Peripheral nerve thickening normally occurs after anesthetic macules have appeared. Nerve involvement follows a characteristic pattern of distribution and is more marked in multibacillary cases.
Smear TestThe smear test has a specificity of 100% and a sensitivity of 50%. A smear can be obtained from nasal mucosa, an ear lobe, and/or skin lesions.25–27 Ziehl-Neelsen stain is used to visualize mycobacteria. Ridley's logarithmic scale, or bacterial index, is used to interpret the smear test results, which are recorded as a number followed by a plus mark to express the degree of abundance or scarcity of bacteria per field. The gold standard continues to be histopathology.
Skin BiopsyA skin lesion is biopsied and stained using the Fite-Faraco technique. At the tuberculoid pole, bacilli are not observed; rather, the usual finding will be granulomas, commonly with nerve involvement. Cases that tend toward the lepromatous pole show an inflammatory infiltrate with Virchow cells replete with bacilli and loss of adnexal structures. In cases at the tuberculoid pole, the granulomas contain epithelioid cells, giant Langerhans cells, and a lymphocytic infiltrate.
Reaction to Intradermal Lepromin InjectionThe lepromin test uses inactivated M leprae extracted from lepromas. After intradermal injection of 0.1mL of the antigen (lepromin) in the flexor surface of the forearm, the reaction is interpreted at 2 moments. One inspection looks for an early (Fernández) reaction and the other for a late (Mitsuda) reaction. The Fernández reaction has good sensitivity but cross-reactivity with other mycobacteria is known to occur. This reaction is read at 24 or 48hours. The Mitsuda reaction is read at 21 days and indicates resistance to the bacillus. A nodule measuring more than 5mm indicates positivity. It is important to remember that these tests are not diagnostic; rather, they are used for classification and prognostic purposes. Under the global initiative for eradicating leprosy in countries where the disease is endemic, diagnosis is based on clinical signs and smear tests, even though more sophisticated tools, such as serology, have become available.
SerologyCurrently, diagnosis can be based on the phenolic glycolipid 1 (PGL-1) antibody titer and on polymerase chain reaction (PCR). PGL-1 antibody detection is useful in multibacillary cases but is of little use in paucibacillary patients.28–30 PCR detection of the bacillus is highly specific and sensitive, but the cost of this technique and the required infrastructure stand in the way of routine use.
TreatmentThe elimination of leprosy as a world health problem is feasible, as this infectious disease is one of the few that meet certain strict requirements for eradication. Among the requirements leprosy meets are its spread by a single means of transmission (from untreated infected individuals) and the possibility of diagnosis by means of simple, practical tools. Furthermore, effective therapy is available and once prevalence falls below a certain level in a population, the likelihood of resurgence is very remote. Finally, unlike the situation with tuberculosis, leprosy infection does not seem to be unfavorably influenced by human immunodeficiency virus infection. By 2003 leprosy had been eliminated from 117 countries, but the disease continues to present a public health problem in 17 countries.2 In 1981 the WHO introduced multidrug therapy with rifampicin, clofazimine, and dapsone (diaminodiphenyl sulfone) for first-line treatment.31 All patients should receive this drug combination monthly under supervision (Table 2).
Treatment Stipulated by the World Health Organization.
| Presentation | Monthly, Supervised | Daily | Duration |
| Paucibacillary | Rifampicin 600mg | Dapsone 100mg | 6mo |
| Multibacillary | Rifampicin 600mg | Clofazimine 50mg | 12mo |
| Clofazimine 300mg | Dapsone 100mg | ||
| Single-lesion paucibacillary | Rifampicin 600mg | Single dose | |
| Ofloxacin 400mg | |||
| Minocycline 100mg |
Source: World Health Organization43
Minocycline, ofloxacin, and clarithromycin are among the drugs used as second-line treatments. The strengths of multidrug therapy are the prevention of resistance to dapsone, the rapid decline of infectivity of infected individuals, and the low rate of recurrence and reactions.32 Nonetheless, this treatment period is long and presents logistical problems; adherence is difficult to achieve.
First-Line DrugsThe antibacterial action of rifampicin, which is derived from Streptomyces fungi, is based on the inhibition of RNA synthesis. Hepatotoxicity, nausea, vomiting, rash, and fever are among this drug's principal adverse effects. The antibacterial activity of clofazimine is low. Although it is known to bind DNA, its mechanism of action is still poorly understood. This drug is thought to generate cytotoxic superoxide radicals and to have anti-inflammatory properties. Its use is associated with a lower incidence of erythema nodosum. Clofazimine is contraindicated in kidney failure and is associated with changes in skin coloring. Dapsone is a sulfonamide whose antibacterial mechanism relies on p-aminobenzoic acid (PABA) antagonism and inhibition of folate synthesis. It is associated with hemolysis (mainly in patients with glucose-6-phosphate-dehydrogenase deficiency), peripheral neuropathy, and erythema nodosum.33 Resistance to rifampicin and to dapsone has been described in association with the rpoB and folP1 genes, respectively.34 The second-line drugs are highly active, but their cost prohibits their use as first-choice treatments.
Management of Leprosy ReactionsA type 1 leprosy reaction, which usually occurs in the first 2 months of starting treatment, presents with erythema, edema, and neuritis. Neuritis is managed with prednisone at a dosage of 40 to 60mg/d; ideally, therapy should be withdrawn after a few weeks.2,35,36 Erythema nodosum is accompanied by fever, nodules, skeletal pain, neuritis, and dactylitis. This condition usually develops between the first and second year and there are intermittent relapses. The treatment of choice is thalidomide, but prednisone or clofazimine can sometimes be prescribed. Thalidomide is started at a dosage of 100 to 200mg/d, although starting dosages as high as 400mg/d have been described; treatment is ideally withdrawn after 3 to 4 weeks.37,38 On relapse, the treatment period can be lengthened, but adverse neuropathic and teratogenic effects should be watched for. Thalidomide's mechanism of action is still poorly understood, but it is known to inhibit tumor necrosis factor. Clofazimine is a good choice for its anti-inflammatory effect, and it can be prescribed at a dosage of 300mg/d in women of childbearing age or in patients who cannot tolerate thalidomide.
Follow-UpA complete physical examination and smear test should be scheduled every 6 months while multibacillary cases are being treated. Histopathology should be performed at the end of each treatment cycle. In paucibacillary cases, histopathology is performed only at the end of treatment.
Preventing DisabilityPatient education is a crucial aspect of treatment. It is important to avoid the stigmatizing of patients with this disease, emphasizing that leprosy is not highly contagious. An informed patient will take greater responsibility for following treatment. It is also necessary to insist that deformity is preventable. An epidemiologic study of patients with leprosy in Ethiopia found disability in 61.5%.39
Like diabetes mellitus, leprosy causes neuropathy, and appropriate care is required to prevent disability. Thus, the prevention of sequelae is an important part of the therapeutic agenda for patients with leprosy. Particular care should be taken to avert trauma and microtrauma to the extremities, especially the feet.15 The patient should be examined periodically and repeatedly, and taught about appropriate footwear and how to care for the feet. Ulcers that develop secondary to leprosy improve when pressure is eliminated. Leprosy should not be a life-threatening disease at this time. When death occurs, it is the result of secondary infections (pneumonia and tuberculosis), amyloidosis, and/or kidney failure.40
VaccinesSeveral vaccines have proven effective to one degree or another in countries where leprosy is endemic. The prophylactic effect of a leprosy vaccine is achieved by resetting the immune system against shared mycobacterial antigens. Some of the vaccines currently in use are Mycobacterium w proposed by Talwar in 1978; the Convit vaccine introduced in 1992, which is the Bacillus Calmette-Guérin (BCG) combined with M leprae; and Mycobacterium ICRC (based on Mycobacterium avium-intracellulare). Others are one based on Mycobacterium tufu (proposed by Iushin and Kalianina in 1995) and one using Mycobacterium habana (see Singh and coworkers, 1997).41 The BCG vaccine itself has been reported to confer up to 50% protection against leprosy.42 A study in India found that the combination of BCG with heat-inactivated M leprae conferred 64% protection. In regions where the Mycobacterium w vaccine has been used along with antileprosy treatment in multibacillary leprosy, there have been reports of accelerated clinical regression and improvement in the bacterial index in patients with a partial response to current therapies. In some regions the BCG vaccine is administered to children under the age of 12 years who are in contact with relatives who have leprosy.
ConclusionHansen disease remains a concern today. All physicians must have a basic understanding of this disease in order to diagnose it and prevent disability and/or contagion. Knowledge of immunopathologic mechanisms reveals the complexity of certain diseases and provides the basis for understanding and treating them. Our current level of knowledge makes it possible to eliminate leprosy, a goal that calls for the concerted efforts of medical, social, political, and scientific resources to prevent the spread of an infection that should no longer exist.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We wish to thank Dr Rodrigo Cepeda Valdés for his valuable contribution of reviewing the literature for this article.
Please cite this article as: Eichelmann K, et al. Lepra: Puesta al día. Definición, patogénesis, clasificación, diagnóstico y tratamiento. Actas Dermosifiliogr. 2013;104:554–63.