The skin can be key to early diagnosis of systemic malignancies. In the second part of this review, we present various skin conditions that can, in certain contexts, reveal the presence of malignancy. The skin conditions are presented in groups based on a diverse range of morphological characteristics. Specifically, the following groups are analyzed: erosive and blistering lesions; inflammatory papules and nodules; xerosis, ichthyosis, and generalized exfoliative dermatitis; symptoms such as pruritus; abnormal hair distribution patterns; sweating disorders; benign tumors that can form part of hereditary syndromes associated with a risk of visceral cancer; and finally, oral and nail abnormalities.
This review highlights the importance of the skin in the study of systemic malignancies.
La piel puede ser “clave” en el diagnóstico precoz de neoplasias sistémicas. En esta segunda parte se abordan una serie de dermatosis agrupadas por sus características morfológicas, muy diversas, que en un contexto apropiado contribuyen a desenmascarar procesos malignos. Se analizan las lesiones erosivas y ampollares, las pápulas y nódulos inflamatorios, la xerosis, ictiosis, dermatitis exfoliativa generalizada, síntomas como el prurito, alteraciones de la distribución del pelo, trastornos de la sudoración, tumores benignos que pueden formar parte de síndromes heredofamiliares en cuya evolución se puede desarrollar cáncer visceral. Por último se describen las lesiones ungueales y bucales en relación con malignidad sistémica.
En definitiva, se destaca la importancia de la piel en el estudio de las neoplasias sistémicas.
The skin should never be overlooked in systemic diseases, as it is perhaps the body's most accessible organ and can be examined using noninvasive techniques.
Furthermore, it can provide the first clues to a diagnosis in 1% of internal malignancies. These clues, combined with a thorough clinical history, can play an important role in alerting the clinician to the presence of an underlying tumor.
Cutaneous manifestations of internal malignancies may be due to direct effects, i.e., invasion of the skin by a tumor or its metastases, or to indirect effects that trigger the cutaneous signs or symptoms but are not a direct manifestation of the primary tumor. While some of these manifestations occur in isolation, others form part of complex paraneoplastic syndromes. In both cases, however, they can indicate the presence of an underlying neoplasm.1–7
In the second part of this review, we will continue with our analysis of cutaneous signs and symptoms that can lead to a diagnosis of an underlying malignancy irrespective of whether or not they form part of a paraneoplastic syndrome. We look at a wide range of conditions, some of which are considered classic paraneoplastic syndromes (marked with an asterisk [*]) and others that correspond to a diverse group of skin conditions frequently seen in routine practice. While this second group of conditions do not normally indicate malignancy, in certain situations and with certain features, they can alert the dermatologist to the presence of an underlying tumor. In all cases, a high index of clinical suspicion is required. In such cases, the dermatologist will be responsible for ordering additional tests, if warranted, and for monitoring the patient. This is particularly important in conditions that are not commonly associated with malignancy or that are associated with a higher risk of certain tumors. We have used 2 asterisks (**) to denote conditions that are occasionally associated with an internal malignancy and 3 asterisks (***) to denote those that are only rarely associated (Table 1).
Possible Cutaneous Signs of Internal Malignancies.
| Facial reddening, swelling, and flushing |
| Hyperpigmentation |
| Annular lesions |
| Erythematosquamous and hyperkeratotic lesions |
| Skin thickening and hardening |
| Vascular lesions |
| Erosive and bullous lesions |
| Inflammatory papules and nodules |
| Xerosis, ichthyosis exfoliative dermatitis (erythroderma) |
| Pruritus and prurigo |
| Hirsutism and hypertrichosis |
| Hyperhidrosis and anhidrosis |
| Cutaneous tumors |
| Mouth changes |
| Nail changes |
The signs and symptoms are classified according to clinical morphologic criteria and listed in random order (Table 1). The first lesions dealt with in the second part of this review are erosive and bullous lesions.
Erosive and Bullous LesionsThe presence of erosions and blisters on the skin and mucous membranes can indicate the presence of conditions such as lichen planus, erythema multiforme exudativum, and autoimmune bullous disorders. These erosive and bullous lesions can sometimes coexist in certain conditions (e.g., paraneoplastic pemphigus8,9) in association with an underlying malignancy, affecting both children10 and adults (Table 2).
Skin Disorders With Erosive and Bullous Lesions Potentially Associated With an Internal Malignancy.
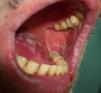
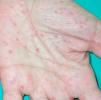
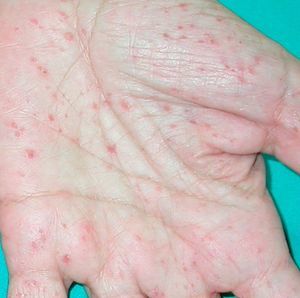
Paraneoplastic pemphigus has certain features that distinguish it from pemphigus vulgaris, namely, persistent severe, painful stomatitis; involvement of other mucosa; and, in certain cases, the presence of polymorphous, vesicular, lichenoid skin eruptions that sometimes mimic the lesions seen in erythema multiforme exudativum, toxic epidermal necrolysis, erythroderma, or lichen planus. Acral lesions affecting the palms and soles and paronychia are common in paraneoplastic pemphigus but rarely observed in pemphigus vulgaris (Figs. 1 and 2).
Histologic examination of skin biopsy specimens from patients with paraneoplastic pemphigus shows interface dermatitis, vacuolization of the basement membrane, necrotic keratinocytes, suprabasal clefting, and acantholysis. The lichenoid inflammatory infiltrate may also be more predominant than in pemphigus vulgaris. Direct immunofluorescence testing reveals immunoglobulin (Ig) G and C3 deposits in intercellular epidermal spaces and at the dermal-epidermal junction, but negative results are also a characteristic feature of paraneoplastic pemphigus.11 Circulating antiplatelet antibodies are detected in peripheral blood.
In two-thirds of cases, the underlying neoplasm is diagnosed before the onset of pemphigus. The most commonly associated malignancies are non-Hodgkin lymphoma, chronic lymphoid leukemia, and Castleman disease (which is more common in children). Less common malignancies are thymoma, Waldenström macroglobulinemia, lung adenocarcinoma, follicular lymphoma, retroperitoneal liposarcoma, and on occasions, hepatocellular carcinoma.12
In the remaining one-third of cases, the neoplasm appears after the pemphigus lesions, hence the importance of screening for occult disease in patients with persistent mucosal lesions that are refractory to treatment. Respiratory failure due to bronchiolitis obliterans is common—and fatal—in patients with paraneoplastic pemphigus, who may die within a month to 2 years of diagnosis of this lung disease.13,14
The autoimmune bullous diseases listed below may also be paraneoplastic, but their association with underlying neoplasms is a subject of debate as an association has only occasionally been observed and therefore might be coincidental.
- 1.
Bullous pemphigoid (***) occurs in elderly patients at a rate that would be expected for this age group. However, there have been reports of cases refractory to conventional treatment in patients with cancer.
- 2.
Cicatricial pemphigoid (***) has been classified as paraneoplastic in certain cases of antiepiligrin cicatricial pemphigoid.
- 3.
Patients with celiac disease and dermatitis herpetiforme (***), a blistering disease frequently associated with gluten enteropathy, can develop intestinal lymphoma.
- 4.
Linear IgA bullous dermatosis (***) has been described in association with lymphoproliferative diseases and renal cell carcinoma.15–17
- 5.
A diagnosis of epidermolysis bullosa acquisita (***), which is characterized by antibodies to type VII collagen, may indicate the presence of a lymphoreticular or other tumor.18
- 6.
There has been an anecdotal report of pemphigus foliaceus (***) associated with non-Hodgkin lymphoma that responded to treatment with rituximab.19
- 7.
Persistent, atypical erythema multiforme exudativum (***) has been associated with renal cell carcinoma, gastric adenocarcinoma, and extrahepatic cholangiocarcinoma.20
In this section, we will look at skin disorders that are predominantly characterized by the presence of papules, plaques, and nodules occasionally accompanied by inflammation. Some of these conditions, for instance, necrobiotic xanthogranuloma, multicentric reticulohistiocytosis, and Sweet syndrome, are strongly associated with underlying neoplasms. Others, however, such as erythema nodosum and cutaneous sarcoidosis, are more weakly associated21,22 (Table 3).
Skin disorders With Inflammatory Papules and Nodules Potentially Associated With an Internal Malignancy.
Necrobiotic xanthogranuloma is characterized by multiple yellowish plaques and subcutaneous nodules located in the periorbital region and on the head, the neck, the flexures of the extremities, and the trunk. Ulceration and scarring are common. The condition is associated with benign monoclonal gammopathy in 80% of cases. Associated malignancies include lymphoproliferative diseases, myeloma, chronic lymphoid leukemia, and Hodgkin and non-Hodgkin lymphoma.3,23
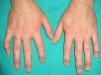
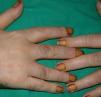
Multicentric Reticulohistiocytosis (**)Multicentric reticulohistiocytosis starts with skin-colored, pink, or brownish papules measuring between a few millimeters and 2cm in diameter located on the dorsum of the fingers, around the nailfold. They have a characteristic coral-bead appearance. The lesions can subsequently spread to other sites, such as the periorbital region, the elbows, the knees, the feet, and the shoulders. Histologic findings include a histiocytic infiltrate with multinucleated giant cells with an eosinophilic cytoplasm and a ground-glass appearance. Patients also develop destructive symmetric arthritis of the hands and knees. Between 20% and 25% of patients have an associated hematologicl malignancy or breast, stomach, ovarian, or cervical cancer.3,23–26
Sweet Syndrome or Acute Febrile Neutrophilic Dermatosis (**)Sweet syndrome is characterized by the sudden onset of asymmetric skin lesions on the face, the extremities, and the upper part of the trunk. The lesions include multiple erythematous or violaceous papules, indurated plaques, and painful inflammatory nodules that are typically accompanied by fever, neutrophilia, and systemic alterations
Skin biopsy shows a dense neutrophilic infiltrate in the papillary dermis, with a variable degree of edema and absence of vasculitis.
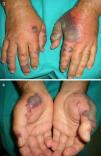
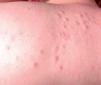
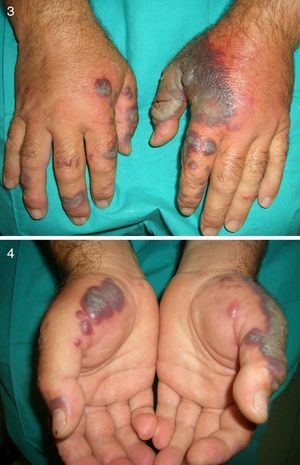
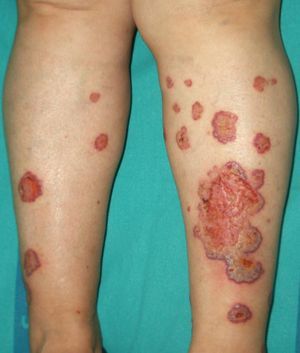
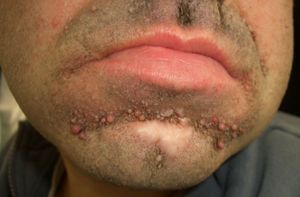
Between 10% and 40% of patients with Sweet syndrome have an associated malignancy, which can precede or arise at the same time as the skin lesions. The most common malignancies are acute myeloid leukemia and other hematological disorders. Solid tumors (genitourinary, breast, lung, and gastrointestinal cancer) are less common. Sweet syndrome is not necessarily associated with malignancy, as it may be idiopathic or induced by other factors such as infectious agents or drugs. There are, however, certain groups of patients in whom it is necessary to rule out an underlying neoplasm. Examples are elderly patients and children without leukocytosis or neutrophilia but with concomitant anemia and thrombopenia; these patients are generally afebrile and have painful lesions on the head, the neck, and the arms; these lesions often take the form of vesicles, pustules, blood-filled blisters, or ulcers, and tend to affect the mucous membranes (Figs. 3 and 4).3,27–31
It has been suggested that pyoderma gangrenosum and Sweet syndrome may be part of the same spectrum. In fact, atypical pyoderma gangrenosum (**), which is an ulcerative neutrophilic dermatosis, is more closely associated with hematological malignancies similar to those seen in Sweet syndrome than classical pyoderma gangrenosum. It is characterized by superficial bullous lesions that form ulcers and are typically located on the head and neck, although they can affect other parts of the body (Figs. 4 and 5).2,3,21,29
Cutaneous Sarcoidosis (***)Cutaneous sarcoidosis has a wide clinical spectrum that includes papules, plaques, nodules, lupus pernio, scar induration, ulceration, alopecia, hypopigmentation, lichenoid or psoriasiform lesions, and more rarely, nail dystrophy or ichthyosiform lesions. Its association with malignancy is attributed to an immune imbalance. The most common associated malignancies are hematological disorders, but a wide variety of solid tumors have also been reported.22
On rarer occasions, other inflammatory lesions such as erythema nodosum (***) may indicate the presence of Hodgkin lymphoma. These lesions are atypical as they last between 1 and 5 months and do not respond to conventional treatment.32
Necrotizing Panniculitis or Subcutaneous Fat Necrosis(**)Necrotizing panniculitis, also known as pancreatic panniculitis as it occurs in pancreatic disorders (including cancer of the pancreas), is caused by the excessive production of pancreatic lipase, an enzyme that induces fat necrosis. It is clinically characterized by the presence of painful erythematous plaques on the buttocks and pretibial regions, although the trunk and the upper limbs may also be affected. The lesions ulcerate, acquiring an abscess-like appearance, and discharge a brownish substance resulting from the liquefaction of subcutaneous fat. Other symptoms, such as fever, arthralgia, and eosinophilia, are also occasionally observed. Patients with necrotizing panniculitis should be evaluated for the presence of acinar cell carcinoma of the pancreas.33
Xerosis, Ichthyosis, Exfoliative Dermatitis (Erythroderma)Xerosis is a common sign that can be constitutional or form part of atopic dermatitis. It may occasionally indicate the presence of an underlying malignancy (Table 4).
Xerosis is common in elderly patients, in whom it typically affects the pretibial region of the lower legs; its severe form is known as eczema craquelé or asteatotic eczema (***). Patients with hormonal disorders and malnutrition are particularly prone to this extreme form of dry skin.
Eczema craquelé has been described in association with lymphomas, gastric adenocarcinoma, glucagonoma, and adenocarcinoma of the pancreas.34 When the lesions acquire a more inflammatory appearance, are more evident on the trunks and upper legs, and furthermore are refractory to treatment with topical corticosteroids, it is advisable to examine lymph nodes, check for hepatosplenomegaly, and perform blood tests, a chest radiograph, and an abdominal ultrasound to rule out the presence of neoplastic disease.35,36
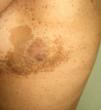
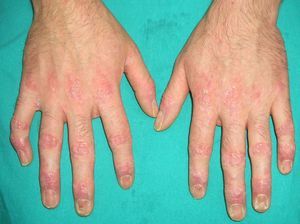
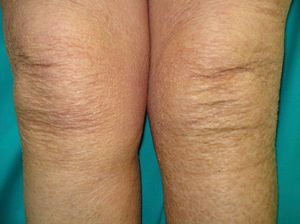
Ichthyosis acquisita (**) is a more severe form of eczema craquelé characterized by small white or brownish rhomboid scales on the extensor surfaces of the extremities and on the trunk. The palms, soles, and flexures are spared (Figs. 5 and 6). Ichthyosis acquisita is mostly seen in association with Hodgkin lymphoma, but it can also occur in association with other lymphoproliferative diseases (e.g., mycosis fungoides, reticulolymphosarcoma, multiple myeloma) and nonlymphoproliferative diseases (ovarian dysgerminoma, leiomyosarcoma, transitional cell cancer of the kidney, hepatocellular carcinoma, breast cancer, and Kaposi sarcoma). One characteristic feature of ichthyosis acquisita is that it typically appears several weeks or months after diagnosis of the tumor. Its pathogenesis is attributed to reduced synthesis of lipids or to an abnormal immune response.3,37–39
Finally, several patients develop, either initially or at a later stage, generalized exfoliative dermatitis (**) with an erythrodermal appearance and severe scaling. They frequently also have alopecia, nail dystrophy, generalized lymphadenopathy, hypothermia, hypoalbuminemia, and heart failure. In most cases, the condition is associated with direct invasion by a cutaneous T-cell lymphoma, but, more rarely, it can occur in association with other lymphomas or with leukemias or solid tumors.32,40
Pruritus and Prurigo (**)Pruritus is a common dermatologic symptom with many causes. In the case of generalized dermatitis, it is important to consider the possibility of an underlying malignancy once the usual benign diseases have been ruled out. Generalized pruritus can be a nonspecific sign of malignancy. It has a complex pathogenesis in which T cell-dysregulation and the production of mediators such as histamine and serotonin by certain tumors has been implicated.2,23
The most common malignancy associated with generalized pruritus is Hodgkin lymphoma; this skin condition is observed in over 25% of patients with this cancer and is refractory to conventional treatments.32 It may also occur in breast cancer, carcinoid syndrome, cutaneous T-cell lymphoma, and gastrointestinal and hepatocellular carcinomas.41
Patients with severe generalized pruritus may have scratch lesions and excoriated papules (prurigo) in accessible areas, namely, the upper third of the back and the arms and legs.23,42
Localized pruritus is very rarely a sign of an underlying malignancy. One example is nasal pruritus, which is a poor prognostic factor in patients with brain tumors.2
Hirsutism and HypertrichosisHypertrichosis refers to the growth of hair in an excessive amount or thickness on any part of the body, while hirsutism refers to the excessive growth of body or facial hair with male characteristics and a male distribution pattern in women (Table 5).
Hirsutism (**) can be caused by the excessive production of hormones by the adrenal or pituitary glands, but it can also be idiopathic. Abrupt onset sometimes suggests the presence of an androgen-producing tumor (typically ovarian) and is accompanied by hormone changes and other signs of virilization.7
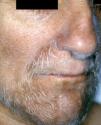
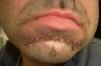
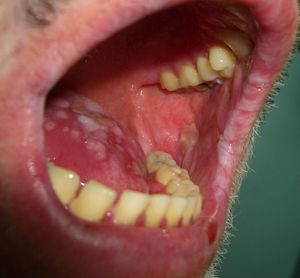
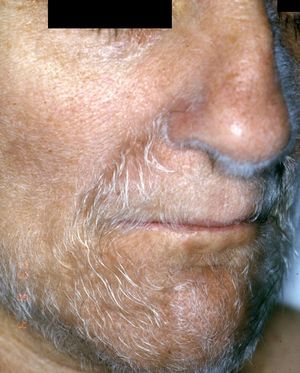
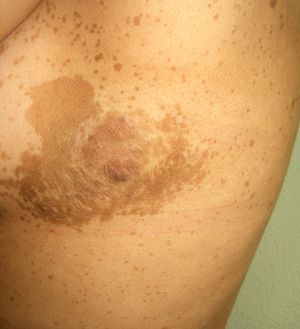
Acquired hypertrichosis lanuginosa is a rare but typically paraneoplastic condition (*) characterized by the sudden appearance of fine, unpigmented hair on the face (Figs. 6 and 7) and then on the trunk, the axillae, and the extremities, with sparing of the palms, soles, and genital area. In women, there are no other signs of virilization. It is strongly paraneoplastic and is more common in women than in men. Accompanying symptoms may include painful glossitis, angular cheilitis, and papillary hypertrophy of the tongue.
In men, acquired hypertrichosis lanuginosa is most commonly associated with lung cancer, followed by colorectal cancer. In women, the most common neoplasms are, in order of frequency, colorectal cancer, lung cancer, and breast cancer. However, the condition has also been described in association with cancers of the ovary, uterus, bladder, pancreas, and kidney, as well as with lymphomas and leukemia. It can precede or follow the diagnosis of the tumor, and is usually a sign of poor prognosis.23,29,43,44
Hyperhidrosis and Anhidrosis (***)Hyperhidrosis has multiple causes and on rare occasions can indicate the presence of an underlying tumor (Table 6).
- 1.
Generalized hyperhidrosis is observed in pheochromocytoma, carcinoid tumors, Hodgkin lymphoma (night sweating), and tumors of the cerebral cortex.
- 2.
Localized craniofacial hyperhidrosis occurs in situations of minimum stress. When it affects a single cheek and is triggered by the ingestion of certain foods and accompanied by salivation, it is known as Frey syndrome, which results from damage to the parasympathetic fibers in the auriculotemporal nerve. Also known as gustatory sweating, it occurs in parotid disorders, which may be neoplastic.45,46
- 3.
Segmental hyperhidrosis is caused by intrathoracic neoplasms that alter the trunk of the sympathetic nerve (lymphomas, mesotheliomas, Pancoast tumor, contralateral lung cancer, or superior mediastinal neurinoma47). The condition is also known as Harlequin syndrome and is characterized by asymmetric flushing and unilateral sweating induced by heat and exercise. It can also be idiopathic.
- 4.
Anhidrosis occurs in neurogenic tumors that affect the central nervous system at the level of the hypothalamus or the spinal cord; it can also occur in association with segmental hyperhidrosis.
Certain benign and malignant tumors are not indicative of malignancy on their own, but they commonly form part of complex syndromes, including certain genodermatoses, that are associated with a higher incidence of neoplasms.6 Familiarity with these syndromes is key to early diagnosis and treatment and consequently improved survival (Table 7).
- 1.
Seborrheic keratoses are very common in elderly patients, but a sudden eruption or increase in the number and size of lesions, accompanied by intense itching, may be indicative of malignancy. This phenomenon is known as the sign of Leser-Trélat (**). The paraneoplastic nature of these lesions, however, has long been the subject of debate as seborrheic keratoses and neoplasms are relatively common in elderly individuals. The sign of Leser-Trélat (**) has been linked to malignant acanthosis nigricans as they sometimes occur together.48 In such cases, the lesions are most often located on the back and can follow a Christmas tree–like pattern. Pruritus is an important symptom. The sign of Leser-Trélat has been associated with gastrointestinal adenocarcinomas and lymphoproliferative diseases, and, less commonly, with lung, bladder, renal, ovarian, and breast cancer.3,23,31,49
- 2.
A similar condition, dermatosis papulosa nigra (**), affects black individuals and has occasionally been linked to malignancy.50
- 3.
Cutaneous florid papillomatosis (*) consists of the development of multiple wartlike lesions on the trunk, face, and extremities. With an identical appearance to that of viral warts, these lesions multiply rapidly and become confluent, occasionally causing unsightly growths and disfigurement of the hands and feet. It is a paraneoplastic disorder that in the opinion of some authors is a clinical variant of malignant acanthosis nigricans.37 It has been described in association with gastric, breast, lung, and ovarian cancer.51–54
- 4.
Multiple sebaceous gland tumors (sebaceous adenomas and carcinomas) located on the trunk are part of Muir-Torre syndrome (**), which runs in families and is associated with carcinomas of the gastrointestinal tract. When there is a single tumor located on the head or neck, it is not usually indicative of malignancy.29
- 5.
The coexistence of trichilemmomas, lipomas, and angiomas suggests a diagnosis of Cowden disease (**) (multiple hamartoma syndrome), a hereditary disorder associated with an increased risk of breast, thyroid, endometrial, lung, and colon cancer.29
- 6.
Gardner syndrome (**), which is characterized by the occurrence of large, multiple epidermal cysts, fibromas, leiomyomas, trichoepitheliomas, and neurofibromas, is an autosomal dominant condition that can occur in association with colon cancer.29
- 7.
Fibrofolliculomas and trichodiscomas are seen in Birt-Hogg-Dubé syndrome (**), an autosomal dominant disorder associated with an increased risk of lung and renal cell carcinoma.29
- 8.
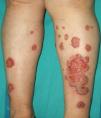
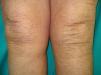
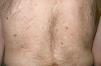
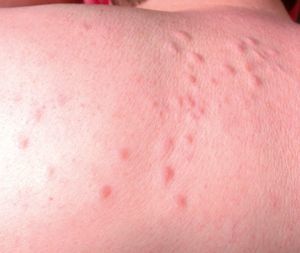
Multiple leiomyomas in a bandlike or segmental distribution (Figs. 7 and 8) may be associated with renal cell tumors, forming part of a syndrome known as hereditary leiomyomatosis and renal cell cancer (**).29
- 9.
Familial atypical multiple mole melanoma (FAMMM) syndrome (***) is a relatively unknown genetic disorder associated with a high risk of melanoma and pancreatic cancer.29
- 10.
Multiple mucosal neuroma syndrome (multiple endocrine neoplasia syndrome) (**) is characterized by the development of multiple neuromas of the lips, the tongue, the lip mucosa, and the gums. It is an autosomal dominant condition associated with a higher incidence of pheochromocytoma and medullary carcinoma of the thyroid.29
- 11.
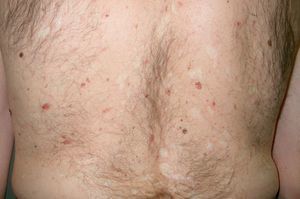
Neurofibromatosis type I (von Recklinghausen disease) (**) is an autosomal dominant neurocutaneous syndrome with freckling in the axillary and groin regions, café au lait spots, neurofibromas, and on occasions, plexiform neurofibromas (Figs. 8 and 9). It can be complicated by the malignant transformation of plexiform neurofibromas. As the disease evolves, patients can develop certain malignancies, such as Wilms tumor, rhabdomyosarcoma, retinoblastoma, melanoma, intestinal leiomyosarcoma, medulloblastoma, and leukemia. Pheochromocytomas are benign in 90% of cases. There have also been reports of familial early-onset breast cancer and other gynecological tumors.2,29
- 12.
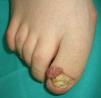
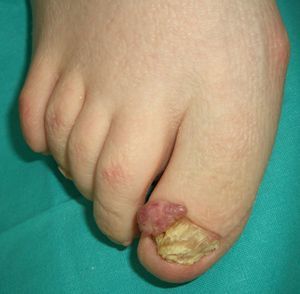
Tuberous sclerosis (**) is an autosomal dominant condition that causes the growth of hamartomas on the skin, the brain, the kidney, and the heart. Characteristic skin lesions include facial angiofibromas (Figs. 9 and 10), periungual fibromas (Koenen tumors) (Figs. 10 and 11), shagreen patches, and hypopigmented ash-leaf macules. The most common tumors in patients with tuberous sclerosis are cerebral astrocytomas, followed by clear cell renal cell carcinomas.2
- 13.
Gorlin syndrome, also known as nevoid basal cell carcinoma syndrome (**), is an autosomal dominant condition consisting of multiple basal cell carcinomas, jaw cysts, palmoplantar pits, and bone anomalies, among other features (Figs. 12 and 13). Patients with this syndrome may also develop other malignancies such as medulloblastomas, oligodendrogliomas, ovarian fibrosarcoma, and Hodgkin and non-Hodgkin lymphoma.2
Cutaneous Tumors Potentially Associated With an Internal Malignancy.
| Seborrheic keratosis (sign of Leser-Trélat)** |
| Eruptive dermatosis papulosa nigra** |
| Cutaneous florid papillomatosis* |
| Sebaceous tumors (Muir-Torre syndrome)** |
| Tricholemmomas, lipomas, angiomas (Cowden syndrome)** |
| Epidermal cysts (Gardner syndrome)** |
| Fibrofolliculomas and trichodiscomas (Birt-Hogg-Dubé syndrome)** |
| Leiomyomas (hereditary leiomyomatosis and renal cell cancer syndrome)** |
| Atypical nevi (familial atypical multiple mole melanoma syndrome)** |
| Multiple neuromas (multiple mucosal neuroma syndrome)** |
| Neurofibromas (neurofibromatosis type I)** |
| Angiofibromas (tuberous sclerosis)** |
| Basal cell carcinomas (Gorlin syndrome or nevoid basal cell carcinoma syndrome)** |
The mouth is the site of many manifestations related to several paraneoplastic signs that have already been discussed (Table 8). These include macroglossia (**) in systemic amyloidosis and myeloma; glossitis (*) in migratory necrolytic erythema and acquired hypertrichosis lanuginosa; erosive lesions in paraneoplastic pemphigus (*); wartlike growths and papillomatosis on the tongue and lips (*) in malignant nigricans acanthosis and cutaneous florid papillomatosis51; and mouth ulcers (**) in Sweet syndrome.55 Oral leukoplakia in dyskeratosis congenita56 and pachyonychia congenita57 will be discussed in the following section on nail changes as these are the main manifestations of these conditions.
Nail ChangesNail changes in isolation do not tend to have much relevance in systemic malignancies, but nail dystrophy is a symptom of numerous paraneoplastic conditions, some of which have already been discussed in the first part of this review. These conditions include acrokeratosis paraneoplastica of Bazex (horizontal or longitudinal ridges and total nail dystrophy); pachydermoperiostosis or hypertrophic osteoarthropathy (acropathy); paraneoplastic pemphigus (paronychia and lichenoid nail dystrophy); tuberous sclerosis (Koenen tumors); and, probably other conditions with late onset of nail changes, including carcinoid syndrome, erythema gyratum repens, and generalized exfoliative dermatitis.
In certain syndromes, however, nail dystrophy is a key sign indicating the possible presence of an underlying neoplasm. The most relevant of these syndromes are listed in Table 9.
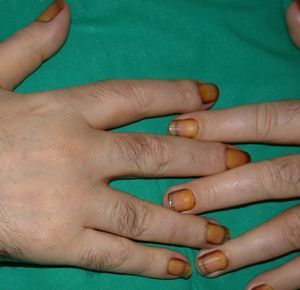
Yellow nail syndrome is a rare condition of unknown etiology, although it is known that hypoplasia of the lymphatic system plays an important role. There have also been occasional reports of the syndrome affecting members of the same family. Yellow nail syndrome is characterized by thick, overcurved greenish nails with loss of cuticle and on occasions the presence of onycholysis (Figs. 13 and 14). Nail growth cessation is not uncommon. The changes can occur alone or in association with a systemic disorder, and it is therefore advisable to rule out respiratory diseases, primary lymphedema, autoimmune diseases such as rheumatoid arthritis, and malignant tumors such as breast cancer and lymphomas.58,59
Dyskeratosis Congenita (**)Dyskeratosis congenita is a multisystemic X-linked recessive disorder characterized by the triad of nail changes, reticulated hyperpigmentation, and leukoplakia of any of the mucous membranes, although the oral mucosa is the most frequently affected.
Nail changes occur early on in the disease. The nails are initially fragile and uneven. They remain small and can even be absent or barely noticeable. The changes first affect the fingernails and then the toenails.
Patients with dyskeratosis congenita have a high risk of aplastic anemia, myelodysplastic syndrome, leukemia, and solid tumors. Leukoplakia, in turn, is associated with a higher incidence of squamous cell carcinoma of the mouth, the rectum, the cervix, the vagina, the esophagus, and the skin.56
Pachyonychia Congenita (**)Pachyonychia congenita refers to a group of ectodermal dysplasias with an autosomal dominant pattern of inheritance. There are 2 main types: pachyonychia congenita type 1 (Jadassohn-Lewandowsky) and pachyonychia congenita type 2 (Jakson-Lawler). Type 1 is characterized by palmoplantar keratoderma, follicular keratosis of the knees and elbows, and oral leukokeratosis and requires close follow-up to check for the development of squamous cell carcinoma. Pachyonychia congenita type 2, in turn, is characterized by steatocystoma multiplex and vellus hair cysts, in addition to many other alterations.
Both types are characterized by considerable thickening of the nails due to subungual hyperkeratosis in the distal portion. The nails acquire a yellow-grayish color and have transverse overcurvature. Nail dystrophy develops during childhood.57
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Yuste Chaves M, Unamuno Pérez P. Alertas cutáneas en malignidades sistémicas (parte 2). Actas Dermosifiliogr. 2013;104:543–53.