LEOPARD syndrome is an autosomal dominant disease caused by germline mutations in the RAS-MAPK (rat sarcoma mitogen-activated protein kinase) pathway. LEOPARD is an acronym for the main manifestations of the syndrome, namely, multiple Lentigines, Electrocardiographic conduction abnormalities, Ocular hypertelorism, Pulmonary stenosis, Abnormalities of genitalia, Retardation of growth, and sensorineural Deafness. None of these characteristic features, however, are pathognomonic of LEOPARD syndrome, and since they are highly variable, they are often not present at the time of diagnosis. We describe 2 cases of LEOPARD syndrome without hearing loss or pulmonary stenosis in which diagnosis was confirmed by identification of a mutation in the PTPN11 gene. Regular monitoring is important for the early detection of complications, as these can occur at any time during the course of disease.
El síndrome LEOPARD es una enfermedad autosómica dominante producida por mutaciones germinales en la vía RAS-MAPK. El acrónimo agrupa las manifestaciones más importantes de la enfermedad (Lentiginosis, ECG conduction anomalies, Ocular hypertelorism/hypertrophic Obstructive cardiomyopathy, Pulmonary stenosis, Abnormalities of genitalia, growth Retardation and Deafness), pero ninguna de ellas es patognomónica ni constante, por lo que muchos pacientes no las presentan en el momento del diagnóstico. Presentamos 2 casos de síndrome LEOPARD sin sordera ni estenosis pulmonar en los que la detección de la mutación en el gen PTPN11 permitió confirmar la enfermedad, y señalamos la importancia del seguimiento continuado para la detección precoz de las complicaciones, ya que las mismas pueden aparecer en el transcurso de la enfermedad.
LEOPARD syndrome, which is currently also called Noonan syndrome with multiple lentigines (NSML) (Online Mendelian Inheritance in Man 151100),1 is an autosomal dominant disease of the RAS (rat sarcoma) gene signaling pathway. The acronym refers to the main manifestations of the syndrome, namely, lentigines, electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormal genitalia, retarded growth, and sensorineural deafness.2,3 These signs are not constant, however, and none of them are pathognomonic. We report 2 genetically confirmed cases of NSML without hypertrophic cardiopathy, pulmonary stenosis, or deafness.
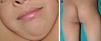
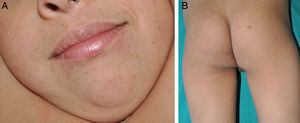
Patient 1A 6-year-old boy, the firstborn of healthy nonconsanguineous parents, had been followed by the neurologist since the age of 4.5 years because of developmental delays, including slight speech-language impairment. Physical examination revealed facial dysmorphism with large, prominent, low-set, posteriorly rotated ears; widely spaced eyes; and a flat nasal bridge. On the back of his neck (area of the cervical spine), right buttock, left axilla, and right knee, the boy also had 4 hyperpigmented macules with irregular borders measuring 5to 15mm in diameter. Multiple lentigines 5mm in diameter were observed on the face, trunk, and extremities (Fig. 1). Cardiac evaluation revealed a right bundle-branch block. A hearing test was normal. Testing for mutations in the PTPN11 (protein tyrosine phosphatase nonreceptor 11) gene demonstrated a T468M mutation (p.Thr468Met) in exon 12.
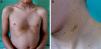
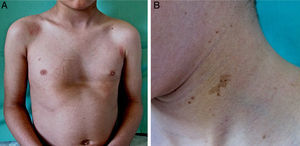
Patient 2A 6-year-old boy born to healthy nonconsanguineous parents was being treated by a speech therapist for delayed language acquisition and difficulty with auditory discrimination of some phonemes. Physical examination revealed facial dysmorphism, including an inverted triangular-shaped face, short neck, large low-set ears, orbital hypertelorism, megalocornea, and pectus excavatum. Numerous café au lait macules and lentigines were present on the face, neck, and upper trunk (Fig. 2). Hearing and cardiac evaluations were normal. Genetic testing detected the R498W mutation (p.Arg498Trp) in axon 13 of PTPN11.
Patient 2. A, Pectus excavatum, a skeletal abnormality that is common in patients with mutations in the RAS-MAPK (rat sarcoma mitogen-activated protein kinase) pathway; hyperpigmented lenticular lesions on the trunk. B, Hyperpigmented macule with irregular borders along with multiple lentigines on the left side of the neck.
NSML belongs to a group of neuro-cardio-fascio-cutaneous syndromes in which germline mutations in the RAS-MAPK (mitogen-activated protein kinase) pathway are present. The most characteristic dermatologic findings are pigmented lesions, especially lentigines, which are present in 90% of patients with these syndromes. Manifestations may be present from birth, but they usually appear when the child is around 4or 5years of age and may even first present in puberty.2,4,5 Café au lait macules, which are somewhat larger than lentigines, can be seen in 70% to 80% of patients. Lesions that are darker have been called black coffee macules. The most common cardiac finding in NSML is hypertrophic myocardiopathy, which can be fatal. Cardiac valve and coronary abnormalities may also be present. Pulmonary stenosis is less common than mitral valve or aortic abnormalities.2 Typical electrocardiographic signs are those of left or right ventricular hypertrophy, right atrial enlargement, and gradual development of right bundle-branch block. Deafness, traditionally considered highly specific to NSML, only presents in 25% to 30% of these patients; the deficit is neurosensorial, may be unilateral or bilateral, and is sometimes profound (>95db). Although deafness is usually diagnosed in childhood, it may develop later, making periodic hearing tests advisable.4
Other manifestations include distinctive facial features; skeletal abnormalities such as pectus carinatum or excavatum, which are seen in up to 75% of neonates; delayed puberty and late menarche; and urogenital abnormalities such as horseshoe-shaped kidney and hypospadia, underdeveloped ovaries or testicles, and small penis.2,4 The slow growth observed in 25% of patients does not appear to be related to endocrine dysregulation or systemic disease. Eighty-five percent of patients will remain in the lower 25th percentile on growth charts.2 Unlike other RASopathies, NSML seems to confer only minimal predisposition to cancer, but blood tumors6 and other malignancies, such as melanomas7,8 have been reported.
Genetic testing is necessary for a definitive diagnosis. Mutations in exons 7, 12, and 13 in the PTP domain of the PTPN11 oncogene that encodes the SHP-2 phosphatase have been demonstrated in 85% to 90% of patients with NSML. Functional SHP2 deficiency (not present in Noonan syndrome) is compensated for by hypersensitivity to growth factor stimulus, longer contact time at the C-terminal domain (pTyr ligands), and the weakening of interactions of the SH2 inhibitor with the catalytic zone.9 The result is functional gain.1 At least 11 different mutations have been described to date. Among them are 2 frequently recurring ones—Y279C and T468M—that have been found in over half of patients with NSML.2,9,10 Of patients who have been found to be negative for PTPN11 mutation, a third have RAF1 (Raf-1 proto-oncogene, serine/threonine kinase) mutations and fewer than 5% have BRAF (B-Raf) mutations.7 There is a certain correlation between genotype and phenotype, such that patients who are negative for PTPN11 mutation tend to have a higher prevalence of cardiac conduction abnormalities, left ventricular or atrial hypertrophy, and a family history of sudden death, whereas patients who are PTPN11-mutation positive (exon 13) have greater risk of hypertrophic myocardiopathy and severe cardiac complications; mutation in exon 7 is more often associated with delayed growth and deafness, and BRAF mutations confer greater risk of cognitive disorders.7RAF mutation may cause lentigines, café au lait macules, abnormal facial features, hypertrophic myocardiopathy, arrhythmias, and delayed puberty.2,4
The main differential diagnoses are other diseases linked to the RAS-MAPK pathway,11 and the strong genetic-phenotypic overlap of RASopathies makes clinical diagnosis difficult. NSML can be phenotypically very difficult to distinguish from Noonan syndrome itself (without multiple lentigines) and neurofibromatosis type 1.12 Lentigines and deafness are not present in Noonan syndrome, and café au lait macules and hypertrophic myocardiopathy are more typical of NSML than of Noonan syndrome.4,13
In conclusion, the 2 cases we report show that NSML cannot be ruled out when deafness and cardiopathy are absent. Genetic testing of these patients is highly advisable, because the findings provide a basis for guiding follow-up and establishing prognosis. However, regardless of which exon is mutated, all patients with NSML should receive periodic evaluations to detect late development of neurologic, dermatologic, cardiac, or hearing complications.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Please cite this article as: Ramos-Geldres T, Dávila-Seijo P, Duat-Rodríguez A, Noguera-Morel L, Ezquieta-Zubicaray B, Rosón-López E, et al. Síndrome LEOPARD sin sordera ni estenosis pulmonar: a propósito de 2 casos. Actas Dermosifiliogr. 2015;106:e19–e22.