Dermoscopy is a noninvasive technique that improves accuracy in the diagnosis of cutaneous lesions. The recognition and differential diagnosis of lentigo maligna (LM) and lentigo maligna melanoma (LMM) is challenging, especially in the early stages when there are no distinctive clinical features. Early diagnosis and appropriate treatment can improve prognosis. Several dermoscopic features have been described for LM and LMM. The following 4 criteria in combination have achieved a diagnostic sensitivity of 89% and a specificity of 96%: asymmetric pigmented follicular openings, dark rhomboidal structures, slate gray dots, and slate gray globules. A biopsy is warranted when dermoscopic examination reveals a grayish coloring. For a flat pigmented lesion acquired in adulthood, a histopathological diagnosis of “atypical junctional nevus” is not to be accepted uncritically. LM and LMM can also appear in sites other than the face, and dermoscopy can facilitate their recognition. Dermoscopy is an essential tool for physical examination.
La dermatoscopia es una técnica no invasiva que aumenta la precisión en el diagnóstico de los tumores cutáneos. El reconocimiento y diagnóstico diferencial del lentigo maligno (LM)-lentigo maligno melanoma (LMM) es desafiante, sobre todo en etapas iniciales, cuando no presenta particularidades clínicas. El diagnóstico precoz posibilita un tratamiento oportuno y adecuado, y podría mejorar el pronóstico. Se describieron múltiples características dermatoscópicas para el LM-LMM. La combinación de 4 criterios otorga una sensibilidad del 89% y una especificidad del 96% para el diagnóstico: pigmentación asimétrica de aperturas foliculares, estructuras romboidales oscuras y puntos y glóbulos gris pizarra. La detección de color gris mediante dermatoscopia amerita la realización de una biopsia. Ante una lesión plana y pigmentada adquirida en la edad adulta no debería plantearse el diagnóstico histólogico de nevo juntural con atipia. El LM-LMM también puede aparecer fuera del rostro, y la dermatoscopia ayuda a reconocerlo. Es fundamental el uso de este instrumento al examinar a nuestros pacientes.
As a noninvasive technique that improves diagnostic accuracy in patients with skin tumors, dermoscopy has become an essential tool in the clinical toolbox.1 Lentigo maligna- lentigo maligna melanoma (LM-LMM) is a subtype of melanoma that arises in skin with chronic sun damage and generally affects the face and scalp of elderly patients. Distinguishing it from other flat pigmented lesions (mainly solar lentigo or initial seborrheic keratosis, pigmented actinic keratosis, and lichenoid keratosis) continues to be a major diagnostic challenge.1,2 Clinical findings alone can be insufficient, particularly in the case of small or early lesions. A correct diagnosis, however, is important, as these entities, while clinically similar, differ significantly in terms of biologic behavior, prognosis, and treatment.3 Familiarity thus with the dermoscopic features of LM-LMM and other entities in the differential diagnosis is very important for ensuring an accurate diagnosis.
Dermoscopic Features of Pigmented Lesions on the FacePigmented lesions on the face have different dermoscopic features to those in other parts of the body. Although the pigment network is a classic dermoscopic feature of melanocytic lesions, it is very rarely found in facial lesions.1,4 The network is formed by melanin in melanocytes or keratinocytes distributed along the long rete ridges of the epidermis. The holes and lines that form this network correspond to the tips and sides of the ridges, respectively. Facial skin with chronic sun damage, however, has a different architecture: it has a flat dermal-epidermal junction and may even have no rete ridges. In such cases, when viewed under a dermoscope, pigmented keratinocytes or melanocytes are seen as diffuse brown areas interrupted by hypopigmented holes of varying width, creating a “pseudonetwork”. These holes correspond to hair follicle and sweat gland openings on the skin surface. This pseudonetwork is observed in melanocytic and nonmelanocytic pigmented lesions on the face. Diagnosis thus is based on the detection of additional criteria, and while the pseudonetwork is specific to the face, it does not offer any information on the lesion's histologic subtype (Fig. 1).1,4
In addition, facial skin is fine and translucent, facilitating the identification of subtle dermoscopic structures, such as pigmentary incontinence and vascular structures.5
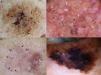
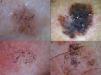
Dermoscopic Features of LM-LMMThe dermoscopic features of LM-LMM were first described by Schiffner et al.,6 who analyzed 87 pigmented lesions on the face to assess the usefulness of dermoscopy in differentiating LM from solar lentigo and initial seborrheic keratosis. Using univariate analysis, they determined 2 specific features of LM: asymmetric pigmented follicular openings and dark (brown or black) rhomboidal structures. The multivariate logistic regression analysis showed that the combination of 4 features—asymmetric pigmented follicular openings, dark rhomboidal structures, slate-gray dots, and slate-gray globules—had a sensitivity of 89% and a specificity of 96% for the diagnosis of LM. The presence, or absence, of 1 of these features does not reliably indicate a diagnosis of LM, as the features are also found in benign lesions (Table 1). The authors also studied correlations with histologic findings, and proposed a progression growth model for LM. The first feature observed, asymmetric pigmented follicular openings, corresponds to the unequal descent of LM cells in individual hair follicles (Fig. 2A). The next feature is formed by short lines produced by the tumor cells in the epidermis or the upper dermis, which merge to form rhomboidal structures as the lesion advances (Fig. 2B). The slate-gray dots and globules reflect clusters of melanophages in the upper dermis whose distribution around the follicular openings create an annular-granular pattern (Fig. 2C). Finally, the pigmented structures merge to completely obliterate the follicular openings, indicating progression to LMM (Fig. 2D). This pattern may also feature zones with whitish scar-like areas and/or milky-red areas (Fig. 2D).1,4,6
Dermoscopic Features of Flat Pigmented Facial Lesions.
| Lentigo Maligna-Lentigo Maligna Melanoma | Solar Lentigo/Flat Seborrheic Keratosis | Pigmented Actinic Keratosis | Lichenoid Keratosis |
|---|---|---|---|
| Asymmetric pigmented follicular openings Rhomboidal structures Slate-gray dots Slate-gray globules Annular-granular pattern Pseudonetwork Red rhomboidal structures Increased density of the vascular network Target-like pattern Darkening at dermoscopic examination Gray color Obliteration of follicular openings Whitish scar-like areas Milky-red areas | Yellow opaque areas Jelly sign Pseudo-milia-like cysts Moth-eaten border Fingerprint-like structures Pseudonetwork Pseudo-follicular openings Asymmetric pigmented follicular openings | Slate-gray dots Annular-granular pattern Inner gray halo Keratin plugs Pseudonetwork Rhomboidal structures Asymmetric pigmented follicular openings | Localized or diffuse gray or brown dots Gray or brown globules, lines, and rhomboid-like structures Remaining areas of seborrheic keratosis or solar lentigo with their corresponding features |
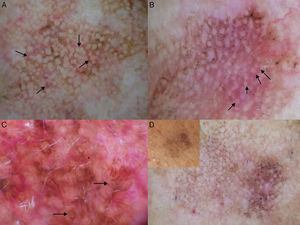
Dermoscopic features of lentigo maligna. A, Asymmetric pigmented follicular openings (black arrows). B, Lines starting to intersect (white arrows) to form rhomboidal structures (black arrows). C. Gray dots (black arrows) forming a granular-annular pattern and asymmetric pigmented follicular openings (white arrows). D, Homogeneous areas with obliteration of the follicular openings (red arrow) and a whitish scar-like area (white arrow).
In a later study, Stante et al.7 found that the Schiffner-Stolz criteria (Fig. 3) could be used for the early detection of LM. They described 4 cases of LM lesions measuring up to 5mm with a homogeneous appearance that had been clinically classified as solar lentigos.7 Blanco et al.8 also demonstrated the value of these criteria in a study of 51 LM-LMM lesions, showing that 81% had at least 1 of the 4 features.8
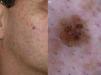
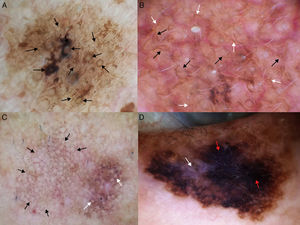
In 2012, Pralong et al.5 described 4 additional dermoscopic criteria for LM-LMM in a study of 125 lesions. Three of the criteria were present in a relatively high proportion of lesions: increased density of the vascular network (found in 58% of lesions), red rhomboidal structures (40%), and target-like patterns (41%). The first feature refers to the observation of a greater density of vessels in the lesion than in the surrounding skin (Fig. 4A). The red rhomboidal structures represent a vascular pattern in the form of a rhomboid around the hair follicles (Fig. 4B). These 2 criteria could be related to tumor neovascularization. The target-like pattern describes a dark dot in the center of a hair follicle with annular hyperpigmentation (Fig. 4C). The fourth criterion, darkening of dermoscopic examination, was observed in 25% of lesions. This feature refers to the fact that the color seen through a dermoscope is darker than that seen in a naked-eye examination (Fig. 4D). Finally, the authors found at least 1 of the 4 classic dermoscopic features in 87% of lesions (a similar proportion to that reported by Ciudad-Blanco et al.8).
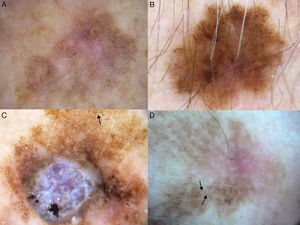
Additional dermoscopic features of lentigo maligna. A, Increased vascular density (black arrows) together with asymmetric pigmented follicular openings and gray dots. B, Red rhomboidal structures (black arrows) and a gray granular-annular pattern. C, Target-like pattern (white arrows) and rhomboidal structures. D, Detection of darker colors in the dermoscopic examination compared with naked-eye inspection (box).
Although the simultaneous presence of 4 or more classic criteria alone has proven to accurately predict a diagnosis of LM, all of the criteria have a common denominator: the gray color. This color is the result of melanin in the upper dermis or in the hair follicles. It can be observed even before the specific dermoscopic structure has formed, and for Zalaudek and her team, it is the single most sensitive finding for the early detection of LM. Its presence indicates the need for biopsy, even in the case of small lesions (Table 1).1–3 In 1 retrospective study of 201 cases of LM, 88.6% of lesions displayed a gray color.9
LM-LMM can also present as an amelanotic or hypomelanotic lesion. In such cases, dermoscopy may reveal red to pink homogeneous areas or a pseudonetwork, dotted vessels and/or atypical linear vessels, whitish structureless areas, and chrysalis (whitish streaks). Traces of pigment may also be detected. These findings can help to distinguish LM-LMM from other nonpigmented lesions, such as actinic keratosis or superficial squamous cell carcinoma.10
Reflectance confocal microscopy (RCM) is another noninvasive technique that has been found to improve the diagnosis and management of facial LM-LMM. RCM provides real-time images of the epidermis and superficial dermis with a cell-like resolution. Guitera et al.11 created an algorithm for differentiating LM-LMM from other pigmented macules of the face with RCM, and reported sensitivity and specificity rates of 85% and 76%, respectively. This method proved equally useful for diagnosing amelanotic and hypomelanotic lesions.11 However, because of its low availability and longer processing times RCM is generally used to evaluate lesions with equivocal findings by dermoscopy rather than to provide an initial diagnostic evaluation.12 One advantage of RCM is that it performs better than dermoscopy for guiding the selection of the biopsy site for subsequent histologic confirmation. It can also help to delineate surgical margins with greater accuracy prior to surgery,11,12 and has even been successfully used to monitor response to nonsurgical treatment (imiquimod, radiation therapy) in patients in whom surgery is contraindicated.13,14 In 1 study, dermoscopy and RCM were used to detect tumor persistence in 98 LM lesions treated with imiquimod or radiation therapy.14 The inflammation and pigmentation secondary to these treatments make it difficult to detect recurrence. Dermoscopy alone was found to have a sensitivity of 80% and a specificity of just 56%. The criterion that was most closely correlated with treatment failure was the presence of very small brown dots with a granular but not an annular pattern. These dots corresponded to pagetoid cells in the epidermis detected by RCM. While asymmetric pigmented follicular openings was a specific finding (present in 81% of lesions), it was found in just 47% of recurrences. RCM, by contrast, showed a sensitivity of 100% and a specificity of 94% for the detection of recurrence.
Extrafacial LM-LMMExtrafacial LM-LMM is rare, and little has been published about this condition. It is important to differentiate extrafacial LM-LMM from superficial spreading melanoma (SSM) in situ, as they are 2 separate entities, with specific clinical, histologic, and even biologic characteristics. Histologically, LM is characterized by a linear proliferation of atypical melanocytes, with nest formation, along the dermal-epidermal junction and on the walls of the hair follicles and sweat glands, in association with epidermal atrophy and solar elastosis.15 The absence of pagetoid spread of melanocytes in LMM is one of the characteristics that differentiates it from SSM.6 One immunohistochemical study showed that SSM in situ cells have greater proliferative activity and higher rates of angiogenesis than LM cells. These findings are consistent with the biologic behavior of these forms of melanoma, as LM has a longer in situ phase than SSM.16 The architectural differences between facial skin and skin in other locations generated interest in whether the dermoscopic features of extrafacial LM would differ from those of facial LM. Carrera et al.17 described a type of early-stage melanoma on the lower limbs with typical dermoscopic features of facial LM. The 5 lesions had light diffuse pigmentation, together with irregular pigmented follicular openings. The histology study showed that the lesions were in situ melanomas with invasion of the follicles by atypical cells. Lau et al.,15 described 3 cases of extrafacial LM, 2 of which had rhomboidal structures and asymmetric follicular pigmentation. Although the lesions also had features of SSM (irregular dots, projections, and an irregular pigment network), the detection of rhomboidal structures and asymmetric follicular pigmentation suggested a diagnosis of extrafacial LM. The presence of classic features of LM can be explained by the involvement of hair follicles in areas other than the face.15
In a recent study, Jaimes et al.18 analyzed the clinical and dermoscopic features of 186 extrafacial melanomas on chronically sun-damaged skin. The lesions were distinct histologic subtypes of melanoma, the most common of which was LM (40.9%), followed by SSM (22.6%). The most common structures were multiple blue-gray dots (67.7%), lines and/or rhomboidal structures (44.1%), and atypical dots or globules (36.6%). Less common findings were the atypical pigment network (29.6%), asymmetric pigmented follicular openings (27.4%), brown areas without a peripheral structure (24.7%), and scar-like areas (19.9%). The authors described 3 patterns that were present in 78% of cases: pigmented islands (reticular areas or patchy peripheral areas), angulated lines (a term they use to describe lines, rhomboidal structures, and a zig-zag pattern), and a pattern consisting of brown structureless areas and blue-gray dots. LM was the most common histologic subtype associated with each one of these patterns (40%-45%). Limitations of the study were that it was retrospective, the evaluators already knew the diagnosis, and the histological findings were not evaluated.18
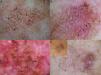
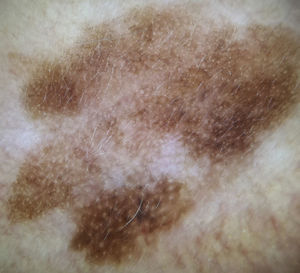
Our group studied 5 cases of extrafacial LM-LMM (4 on the upper limbs and 1 on the trunk). In all cases, we observed gray dots forming an annular-granular pattern and light brown structureless areas (Figs. 5 and 6A-D). We observed asymmetric pigmented follicular openings in 1 case (Fig. 5) and rhomboidal structures in 2 (Fig. 6C, D). Our findings are similar to those of previous reports and we agree that dermoscopy could be useful for diagnosing extrafacial LM and distinguishing it from SSM. This obviously has practical implications, as the diagnostic and treatment approaches differ. More and better-designed studies, with analysis of sensitivity and specificity, are needed.
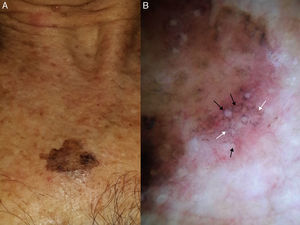
Extrafacial lentigo maligna. A, Irregular dark brown and black lesion, measuring 2cm at its longest point, in the neckline area. B, Dermoscopic features: asymmetric pigmented follicular openings (black arrows), gray dots forming granular-annular pattern (white arrows), and brown structureless areas.
A-D, Dermoscopic features of extrafacial lentigo maligna; lesions located on the upper limbs. Note the gray dots forming a granular-annular pattern in some sectors, the brown structureless areas, and the rhomboidal structures (black arrows). Lesion C is lentigo maligna melanoma; note the whitish-blue veil.
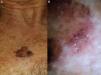
The main entities in the differential diagnosis of LM are solar lentigo and flat seborrheic keratosis. Schiffner and his team observed that pseudocysts, yellow opaque areas, and fingerprint-like structures were suggestive of solar lentigo, together with the jelly sign and the moth-eaten border. The presence of a pseudonetwork should not be confused with the pigment network seen in melanocytic lesions (Fig. 7A). When asymmetric pigmented follicular openings are observed on dermoscopy, it is necessary to perform a biopsy or close monitoring (Table 1). Structures that are very similar to the projections of melanocytic lesions at the border of solar lentigos may be occasionally observed, making it very difficult to differentiate the 2 entities. Pseudofollicular openings and pseudo-milia-like cysts may be seen in thicker seborrheic keratosis lesions (Fig. 7B).4 Melanin is limited to the epidermis (pigmented keratinocytes) in solar lentigo, and is seen as a brown color through the dermoscope; the absence of gray is the most important criterion for distinguishing this condition from LM.1
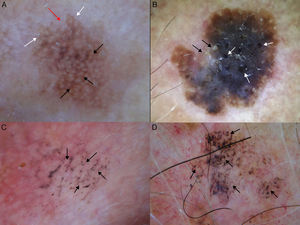
Conditions to be considered in the differential diagnosis of lentigo maligna. A, Solar lentigo: moth-eaten border (red arrow), jelly sign (white arrows), and pseudonetwork (black arrows). B, Seborrheic keratosis: pseudo-milia-like cysts (black arrows) and pseudo-follicular openings (white arrows). C, Pigmented actinic keratosis: gray dots forming a granular-annular pattern (black arrows). D, Lichenoid keratosis: gray dots and globules forming a granular-annular pattern (black arrows).
In pigmented actinic keratosis, melanin is mainly located in the macrophages of the dermis, and dermoscopy shows slate-gray dots (annular-granular pattern) similar to those seen in LM (Fig. 7C). Any of the dermoscopic features of LM can be observed, although asymmetric pigmented follicular openings tend to be absent.4,19 The presence of yellowish or white keratin plugs in the follicles is suggestive of actinic keratosis.1 Nascimiento et al.20 recently described a structure they called the inner gray halo for diagnosing pigmented actinic keratosis. This structure is a gray or beige ring surrounding a hyperkeratotic follicular opening within the brown pseudonetwork (Table 1). They analyzed this feature in 79 lesions (58 pigmented actinic keratosis lesions and 21 LM lesions), and found it to have a sensitivity of 91.4% and a specificity of 71.4%.20 Other diagnostic clues pointing to a diagnosis of pigmented actinic keratosis are the presence of multiple lesions (the neighborhood sign) and a rough surface.4 Histologic evaluation is necessary to distinguish pigmented actinic keratosis from LM in equivocal cases, although it is not always easy to determine whether the atypical pigmented cells at the basal layer are keratinocytes or melanocytes.1
The terms lichenoid keratosis and lichen planus-like keratosis refer to solar lentigo or seborrheic keratosis lesions that have undergone regression. Distinguishing these lesions from LM is problematic and is only possible if some areas of the previously benign lesion remains.21 Lichenoid keratosis that has regressed completely or almost completely is dermoscopically characterized by brown-gray dots, which may merge to form globules, lines, or even rhomboid-like structures (Fig. 7D) (Table 1).21 As these features are also seen in LM, lesions of this type should always be biopsied.1
SSM can also affect the face. In such cases, depending on the location, dermoscopy may show the pseudonetwork, but this may have been destroyed in lesions with rapid horizontal growth. The asymmetric pigmented follicular openings and rhomboidal structures observed in LM are not present in SSM.4
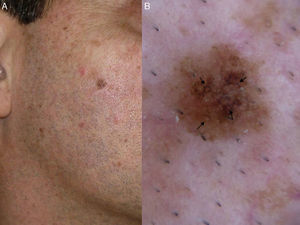
Melanocytic nevus does not need to be considered in the differential diagnosis of flat, pigmented lesions on chronically sun-damaged skin. Nevi located on the head and neck of adults usually presents as hypopigmented cupuliform nodules (Miescher-type intradermal nevi), and are notably different from LM. The validity of a histologic diagnosis of lentiginous nevus or junctional nevus should therefore be considered critically when the biopsy is obtained from a pigmented macule on the face of an elderly patient. This diagnostic error can sometimes occur because early LM may lack significant cytologic atypia or architectural disorder, making it difficult to differentiate from a lentiginous or junctional nevus. Therefore, familiarity with the classic clinical appearance of a facial nevus in an elderly patient will help to prevent an incorrect diagnosis of early LM.2,22
ConclusionsRecognition of LM-LMM and distinction from similar lesions is challenging, particularly in early stages of disease. An accurate diagnosis of LM in small lesions will ensure opportune treatment and possibly improve prognosis. Dermoscopy is a useful tool for the early detection of LM-LMM and should be used to evaluate all facial and extrafacial pigmented lesions. A diagnosis of LM could be missed if evaluation is based only on clinical findings.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bollea-Garlatti LA, Galimberti GN, Galimberti RL. Lentigo maligno. Claves en el diagnóstico dermatoscópico. Actas Dermosifiliogr. 2016;107:489–497.