To perform a cost-effectiveness and cost-utility analysis of ingenol mebutate in the treatment of actinic keratosis in Spain.
MethodsWe used an adapted Markov model to simulate evolution of a cohort of patients (mean age, 73 years) with actinic keratosis over a 5-year period. The comparators were diclofenac 3% and imiquimod 5%. The analysis was performed from the perspective of the Spanish National Health System based on direct costs (2015 retail price plus value added tax less the mandatory discount). A panel of experts estimated resources, taking unit costs from national databases. An annual discount rate of 3% was applied. Deterministic and probabilistic sensitivity analyses were performed.
ResultsThe effectiveness of ingenol mebutate—with 0.192 and 0.129 more clearances gained in treatments for face and scalp lesions and trunk and extremity lesions, respectively—was superior to diclofenac's. The total costs of treatment with ingenol mebutate were lower at € 551.50 (face and scalp) and € 622.27 (trunk and extremities) than the respective costs with diclofenac (€ 849.11 and € 844.93). The incremental cost-effectiveness and cost-utility ratios showed that ingenol mebutate was a dominant strategy vs diclofenac. Ingenol mebutate also proved to be more effective than imiquimod, based on 0.535 and 0.503 clearances, and total costs of € 551.50 and € 527.89 for the two drugs, respectively. The resulting incremental cost-effectiveness ratio was € 728.64 per clearance gained with ingenol mebutate vs imiquimod.
ConclusionsIngenol mebutate was a dominant treatment option vs diclofenac and was efficient vs imiquimod (i.e., more effective at a higher cost, achieving an incremental cost-utility ratio of<€30000/quality-adjusted life-years).
Realizar un análisis coste-efectividad y coste-utilidad de ingenol mebutato en el tratamiento de la queratosis actínica en España.
MétodosSe realizó la adaptación de un modelo de Markov que simuló una cohorte de pacientes (73 años de media) con queratosis actínica en un horizonte temporal de 5 años. Los comparadores fueron diclofenaco 3% e imiquimod 5%. El análisis se desarrolló desde la perspectiva del Sistema Nacional de Salud, incluyendo costes directos sanitarios (PVPIVA con la deducción obligatoria, € 2015). La estimación de recursos se llevó a cabo por un panel de expertos y los costes unitarios se obtuvieron de bases de datos de costes nacionales. La tasa de descuento considerada fue del 3% anual. Se realizaron análisis de sensibilidad determinísticos y probabilísticos.
ResultadosIngenol mebutato fue más eficiente frente a diclofenaco, con 0,192 aclaramientos incrementales en la cara y el cuero cabelludo y 0,129 en el tronco y las extremidades. Los costes totales fueron de 551,50€ y 622,27€ comparados con 849,11€ y 844,93€ en diclofenaco (cara y cuero cabelludo y tronco y extremidades, respectivamente). Es decir, ingenol mebutato es una alternativa de tratamiento dominante frente a diclofenaco 3%. Ingenol mebutato también mostró una mayor eficacia frente a imiquimod 5%, con 0,535 vs 0,503 aclaramientos ganados, y unos costes totales de 551,50€ vs 527,89€, siendo la relación coste-efectividad incremental resultante de 728,64€/aclaramiento adicional.
ConclusionesIngenol mebutato resultó ser una estrategia dominante vs diclofenaco, y eficiente, es decir, presentó mayor efectividad y mayores costes (relación coste-utilidad incremental inferior a 30.000€/AVAC) vs imiquimod.
Actinic keratosis (AK) manifests with red or skin-colored lesions with varying degrees of hyperkeratosis making them feel rough to the touch. Because sun exposure is the main—but not the only—risk factor for AK, the lesions are usually found in exposed areas of the body.1
Fifteen percent of men and 6% of women in Europe develop AK,2 and prevalence rates of 18% in women and 34% in men over the age of 70 years have been reported.3 A Spanish epidemiologic study estimated that AK affects 23.5% of the population over the age of 45 years.4
The risk of progression of AK to invasive carcinoma has been estimated at about 0.75% per lesion per year; an additional 0.53% accrues if there is a history of skin cancer.5 Extrapolating from these data, we can say that an immunocompetent patient with no history of skin cancer who has 8 AK lesions has a 5.8% chance of developing squamous cell carcinoma within 10 years.5
Given the high prevalence of AK, this condition generates a considerable economic burden. In the United States skin cancer is among the 5 most costly cancer diagnoses.6
Besides the carcinogenic potential of AK, the impact of skin diseases on quality of life must be considered.7 Therapies available to treat AK range from minimally invasive ones (such as cryotherapy or pharmacologic approaches) to more radical ones such as shave excision, electrodesiccation and curettage, laser surgery, and chemical exfoliation.8 More recent approaches include the treatment of field cancerization with photodynamic therapy or topical treatments such as 5-fluorouracil (0.5% and 5%), ingenol mebutate (0.015% and 0.05%), diclofenac (3%), and imiquimod (2.5%, 3.75%, and 5%); indications vary according to the area of the body being treated. The aims of these treatments are to eliminate visible lesions (an outcome termed clearance), prevent progression to invasive carcinoma, and halt the development of new lesions. Recurrence is common among treated patients, however.9
Given the budget constraints that have arisen during periods of economic difficulty and the breadth of the therapeutic arsenal at our disposal for AK, there is increased interest in information that can guide the proper selection of treatments and application of resources. Pharmacoeconomics—or the assessment of health technologies—takes the approach of rigorously analyzing the costs and heath benefits of therapeutic alternatives. This approach should be understood as a useful complement to decision-making based purely on clinical information.
This study aimed to estimate the efficiency of ingenol mebutate in the treatment of patients with AK in Spain.
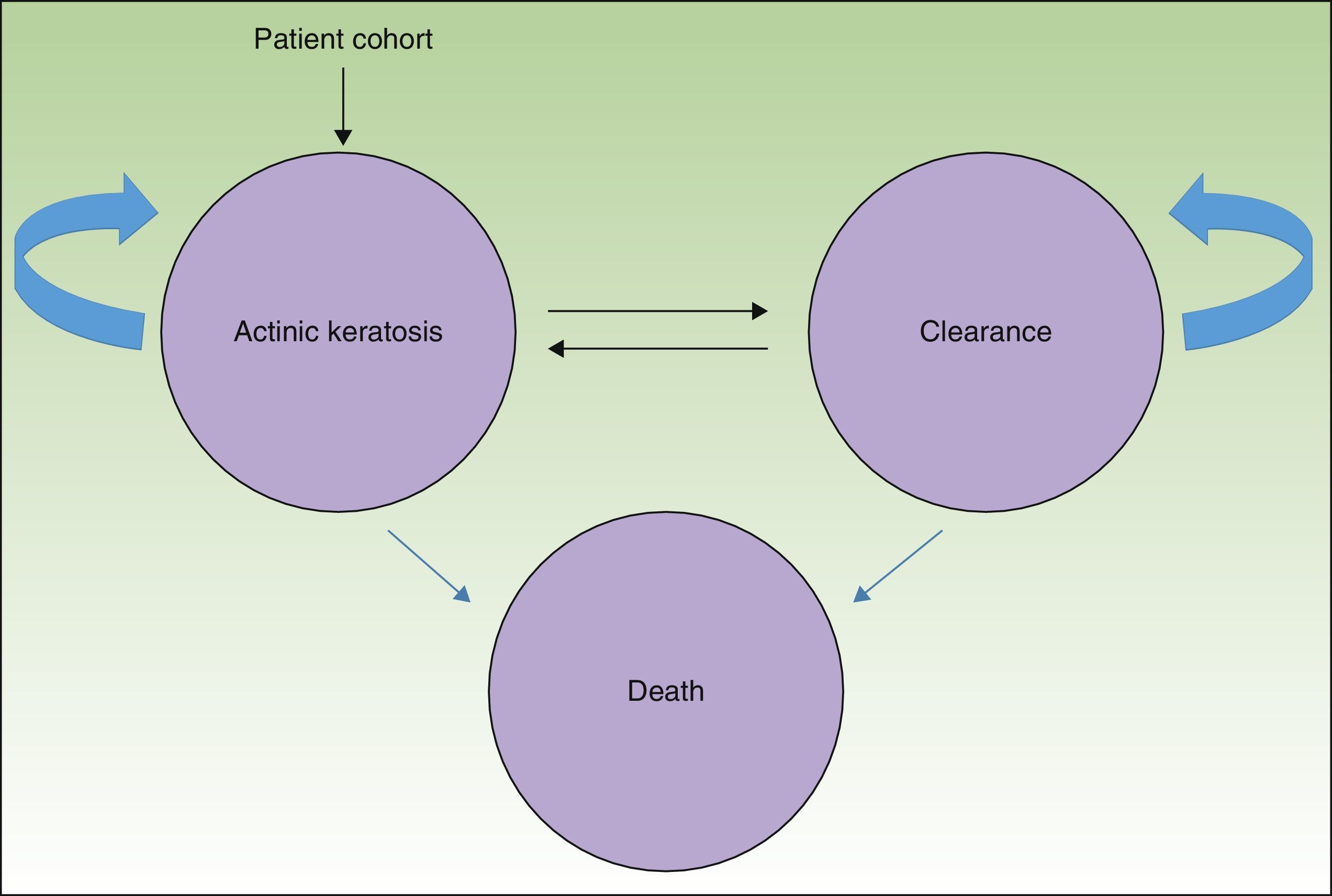
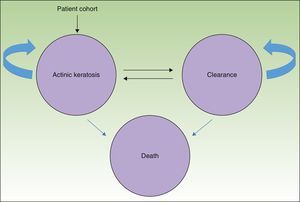
MethodsWe used a Markov model with 3 health states (AK, clearance, and death) (Fig. 1) to simulate the course of disease in a hypothetical patient cohort.
The entire cohort (mean age, 73 years) had AK lesions at the start of the simulation4; potentially they could remain in that state or transition to a state of either clearance or death.
By allowing for changes between the states of clearance and AK, the model recognizes the possibility of recurrence, or the reappearance of lesions. Available evidence estimates an annual recurrence rate of 20%,10 regardless of treatment received.
In this analysis the possibility of death refers not to death caused by AK per se but rather death from any cause occurring in the general population.11
Death was included as a health state in the interest of estimating the efficiency of therapy not only in terms of cost-effectiveness but also cost-utility, which estimates the cost per quality-adjusted life-years (QALY) gained.
This analytical approach was developed with the help of a panel of experts consisting of 5 dermatologists from hospitals in different areas of Spain; these experts are authors of this article. The Delphi method was used to achieve consensus. Questionnaires to collect the information and data needed to execute the model were drafted in Word and Excel and piloted. Responses were compared and discussed at a meeting at which the participants came to agreement about the parameters to include.
Available TherapiesThe comparators were the pharmacologic treatments authorized (in addition to ingenol mebutate) for treating AK on the face and scalp and/or the trunk and extremities: diclofenac 3% and imiquimod 5%.
Given that not all the therapies included are indicated for application on all locations, we posed 3 scenarios: one comparing ingenol mebutate to diclofenac 3% to treat face and scalp lesions, another comparing ingenol mebutate to diclofenac 3% to treat lesions on the trunk and extremities, and another comparing ingenol mebutate to imiquimod 5% for lesions on the face and scalp.
Perspective, Time Horizon, and Discount RateThe perspective adopted was that of the Spanish National Health Service (NHS); therefore, we took only direct health costs into consideration. The time horizon of 5 years was considered sufficient to identify and quantify changes in patients’ conditions, taking into consideration not only the initial treatment but also possible medium-term recurrences. A discount rate of 3% was applied to costs and outcomes.12
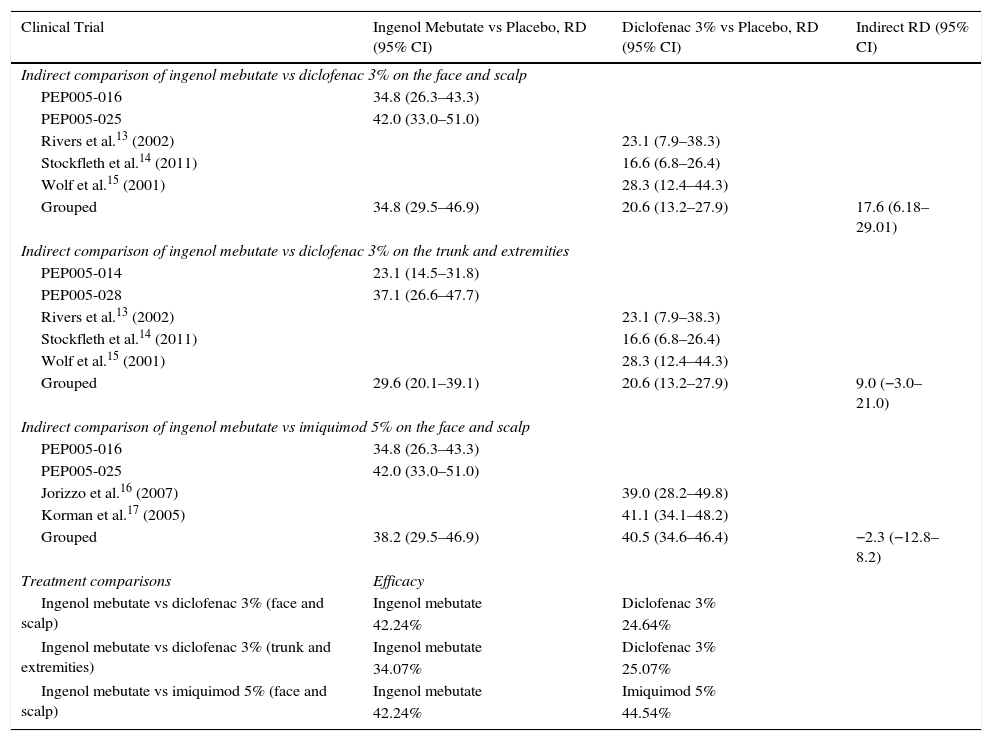
Health OutcomesThe measure of treatment effectiveness was total clearances gained. Because no direct comparisons (head-to-head trials) were available, we obtained data from indirect comparisons (Table 1) using Bucher's method.
Results of Indirect Comparisons of Ingenol Mebutate vs Diclofenac 3% and Imiquimod 5%.
| Clinical Trial | Ingenol Mebutate vs Placebo, RD (95% CI) | Diclofenac 3% vs Placebo, RD (95% CI) | Indirect RD (95% CI) |
|---|---|---|---|
| Indirect comparison of ingenol mebutate vs diclofenac 3% on the face and scalp | |||
| PEP005-016 | 34.8 (26.3–43.3) | ||
| PEP005-025 | 42.0 (33.0–51.0) | ||
| Rivers et al.13 (2002) | 23.1 (7.9–38.3) | ||
| Stockfleth et al.14 (2011) | 16.6 (6.8–26.4) | ||
| Wolf et al.15 (2001) | 28.3 (12.4–44.3) | ||
| Grouped | 34.8 (29.5–46.9) | 20.6 (13.2–27.9) | 17.6 (6.18–29.01) |
| Indirect comparison of ingenol mebutate vs diclofenac 3% on the trunk and extremities | |||
| PEP005-014 | 23.1 (14.5–31.8) | ||
| PEP005-028 | 37.1 (26.6–47.7) | ||
| Rivers et al.13 (2002) | 23.1 (7.9–38.3) | ||
| Stockfleth et al.14 (2011) | 16.6 (6.8–26.4) | ||
| Wolf et al.15 (2001) | 28.3 (12.4–44.3) | ||
| Grouped | 29.6 (20.1–39.1) | 20.6 (13.2–27.9) | 9.0 (−3.0–21.0) |
| Indirect comparison of ingenol mebutate vs imiquimod 5% on the face and scalp | |||
| PEP005-016 | 34.8 (26.3–43.3) | ||
| PEP005-025 | 42.0 (33.0–51.0) | ||
| Jorizzo et al.16 (2007) | 39.0 (28.2–49.8) | ||
| Korman et al.17 (2005) | 41.1 (34.1–48.2) | ||
| Grouped | 38.2 (29.5–46.9) | 40.5 (34.6–46.4) | −2.3 (−12.8–8.2) |
| Treatment comparisons | Efficacy | ||
| Ingenol mebutate vs diclofenac 3% (face and scalp) | Ingenol mebutate | Diclofenac 3% | |
| 42.24% | 24.64% | ||
| Ingenol mebutate vs diclofenac 3% (trunk and extremities) | Ingenol mebutate | Diclofenac 3% | |
| 34.07% | 25.07% | ||
| Ingenol mebutate vs imiquimod 5% (face and scalp) | Ingenol mebutate | Imiquimod 5% | |
| 42.24% | 44.54% | ||
Abbreviation: CI, confidence interval; RD, risk difference.
Clinical trials of AK treatments that used clearance rates as the outcome measure were identified by a search of the literature. Specific trials found for the ingenol mebutate vs diclofenac 3% scenario13–15 were grouped according to part of the body treated (face and scalp or trunk and extremities). In contrast, the trials we found for the scenario comparing ingenol mebutate to imiquimod 5%16,17 only treated the face and scalp because the summary of product characteristics for imiquimod18 does not recognize its use on the trunk and extremities.
The panel of experts consulted during the development process for this study estimated an adherence-to-treatment rate of 90% for ingenol mebutate and 60% for the other treatments; their opinion was that lack of adherence reduced treatment efficacy by 50%.
Measurement of Results ObtainedThe incremental cost-effectiveness and cost-utility ratios (ICER and ICUR, respectively) were calculated as the cost differentials between ingenol mebutate and each comparator divided by the difference in health outcomes between the compared treatments. The denominators for the ICER and ICUR were clearances and QALY gained (ingenol mebutate vs each comparator).
Utility ValuesQALY values were estimated by multiplying life-years gained by a factor termed utility, which represents patients’ preferences for certain states of health. The utility variable was expressed on a scale from 0 to 1, where 1 corresponds to perfect health and 0 represents death.
For this analysis we considered that a value of 0.986 indicated that a treatment was useful for patients with AK; a value of 1 was assigned if complete clearance was observed.19
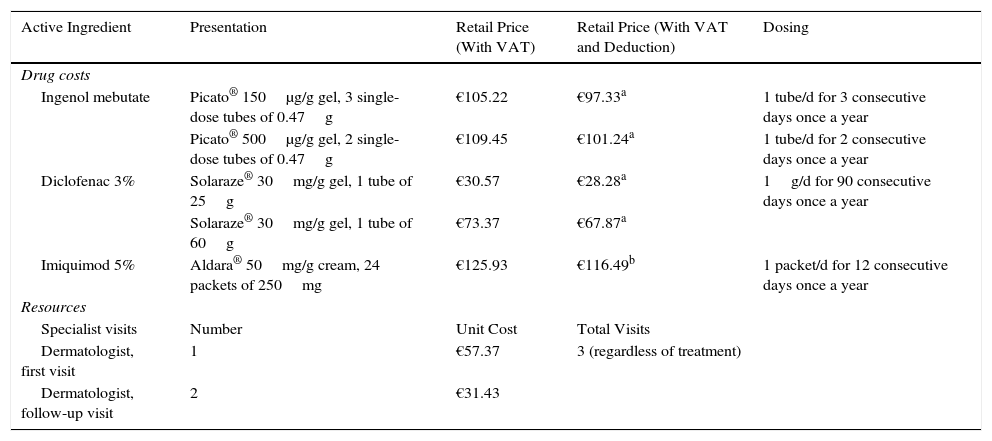
Resource Use and Associated CostsDifferent doses were entered into the analysis of costs according to part of the body being treated (Table 2).
Use of Resources and Spending in Base Cases.
| Active Ingredient | Presentation | Retail Price (With VAT) | Retail Price (With VAT and Deduction) | Dosing |
|---|---|---|---|---|
| Drug costs | ||||
| Ingenol mebutate | Picato® 150μg/g gel, 3 single-dose tubes of 0.47g | €105.22 | €97.33a | 1 tube/d for 3 consecutive days once a year |
| Picato® 500μg/g gel, 2 single-dose tubes of 0.47g | €109.45 | €101.24a | 1 tube/d for 2 consecutive days once a year | |
| Diclofenac 3% | Solaraze® 30mg/g gel, 1 tube of 25g | €30.57 | €28.28a | 1g/d for 90 consecutive days once a year |
| Solaraze® 30mg/g gel, 1 tube of 60g | €73.37 | €67.87a | ||
| Imiquimod 5% | Aldara® 50mg/g cream, 24 packets of 250mg | €125.93 | €116.49b | 1 packet/d for 12 consecutive days once a year |
| Resources | ||||
| Specialist visits | Number | Unit Cost | Total Visits | |
| Dermatologist, first visit | 1 | €57.37 | 3 (regardless of treatment) | |
| Dermatologist, follow-up visit | 2 | €31.43 | ||
The estimated total cost for using each treatment included the retail price of the drug itself plus the cost of visits to a dermatologist for adverse events and follow-up. All costs were expressed in euro values for 2015 (Table 2).
Drug costs were calculated on the basis of the retail price,20 to which we applied the deduction established by Royal Decree-Law 8/2010.21 Resources used (number of visits to the dermatologist during the follow-up period) were identified and their cost estimated based on the judgment of the expert panel (Table 2). Unit costs of the resources used were obtained from published NHS databases.22
Sensitivity AnalysesDeterministic and probabilistic sensitivity analyses were used to study the influence of different parameters on the robutness of the results.
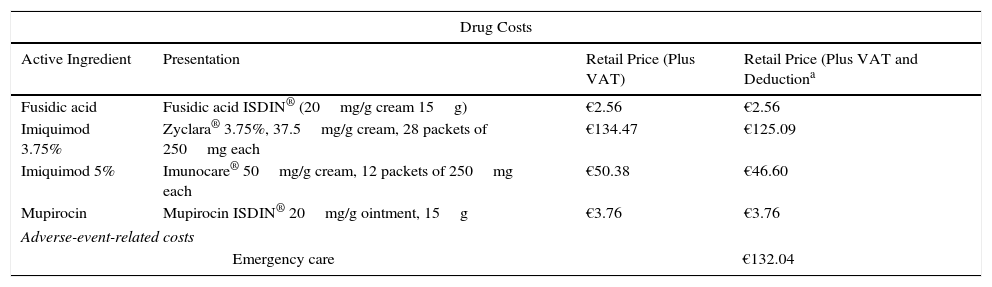
In the deterministic sensitivity analysis the effects of the following parameter modifications were explored: discount rate (0% and 5%), time horizon (30 years), treatment adherence rate (setting the rate of nonadherence set to 10% for all treatments), efficacy (decreasing the value for nonadherence to 33.3%), emergency visits related to adverse events (setting additional emergency visits required to 5% of patients using ingenol mebutate, 2% of those using diclofenac, and 10% of those using imiquimod), additional use of an antibiotic (for patients requiring an additional dermatology visit in 30% of those on ingenol mebutate, 5% of those on diclofenac, and 100% of those on imiquimod), and the use of generic imiquimod and imiquimod 3.75% (including the price differences) (Table 3).
Costs Applied in the Sensitivity Analyses.
| Drug Costs | |||
|---|---|---|---|
| Active Ingredient | Presentation | Retail Price (Plus VAT) | Retail Price (Plus VAT and Deductiona |
| Fusidic acid | Fusidic acid ISDIN® (20mg/g cream 15g) | €2.56 | €2.56 |
| Imiquimod 3.75% | Zyclara® 3.75%, 37.5mg/g cream, 28 packets of 250mg each | €134.47 | €125.09 |
| Imiquimod 5% | Imunocare® 50mg/g cream, 12 packets of 250mg each | €50.38 | €46.60 |
| Mupirocin | Mupirocin ISDIN® 20mg/g ointment, 15g | €3.76 | €3.76 |
| Adverse-event-related costs | |||
| Emergency care | €132.04 | ||
In the probabilistic sensitivity analysis the values for the parameters were modified simultaneously according to the beta, log-normal, and gamma distributions by means of 1000 Monte Carlo simulations.
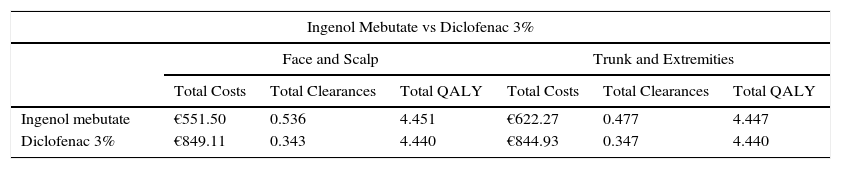
ResultsAt the end of the 5-year simulation, ingenol mebutate had demonstrated more effectiveness than diclofenac 3%. In face and scalp treatments, ingenol mebutate gained 0.192 more clearances and 0.011 QALY more than diclofenac 3%; in trunk and extremity treatments, ingenol mebutate led to 0.129 more clearances than diclofenac 3% and 0.007 additional QALY (Table 4).
Results for Base Case.
| Ingenol Mebutate vs Diclofenac 3% | ||||||
|---|---|---|---|---|---|---|
| Face and Scalp | Trunk and Extremities | |||||
| Total Costs | Total Clearances | Total QALY | Total Costs | Total Clearances | Total QALY | |
| Ingenol mebutate | €551.50 | 0.536 | 4.451 | €622.27 | 0.477 | 4.447 |
| Diclofenac 3% | €849.11 | 0.343 | 4.440 | €844.93 | 0.347 | 4.440 |
| Ingenol Mebutate vs Imiquimod 5% | |||||
|---|---|---|---|---|---|
| Total Costs | Total Clearances | Total QALY | ICER | ICUR | |
| Ingenol mebutate | €551.50 | 0.536 | 4.451 | €728.64/clearance | €10906/QALY |
| Imiquimod 5% | €527.89 | 0.503 | 4.449 | ||
Abbreviations: ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio; QALY, quality-adjusted life-years.
The total 5-year costs were lower with ingenol mebutate than with diclofenac 3%; the incremental costs, and hence savings, were–€297.60 in the treatment of face and scalp AKs and −€222.66 in the treatment of trunk and extremity lesions.
Ingenol mebutate provided better health outcomes in terms of clearances and QALY at lower costs than diclofenac 3% for the treatment of AK on both locations studied.
Ingenol mebutate was also more effective than imiquimod 5%, based on 0.032 additional clearances and 0.002 additional QALY in the treatment of face and scalp AK. The total cost per patient of treatment was €551.50 with ingenol mebutate and €527.89 with imiquimod 5%.
The resulting ICER and ICUR were €728.64/clearance gained and €10906/QALY gained with ingenol mebutate vs imiquimod 5%.
Assuming a-willingness-to-pay threshold of €30000/QALY,23 which is used as a reference value in most economic evaluations of health technologies in Spain,24 treatment with ingenol mebutate would be considered an efficient strategy in comparison with diclofenac 3% or imiquimod 5%.
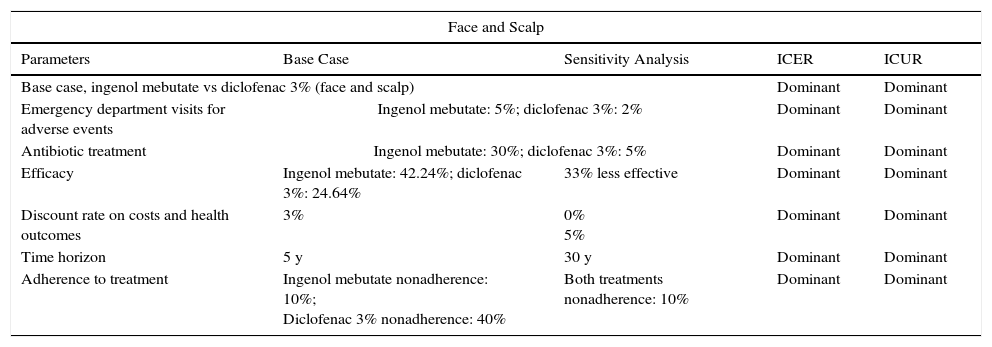
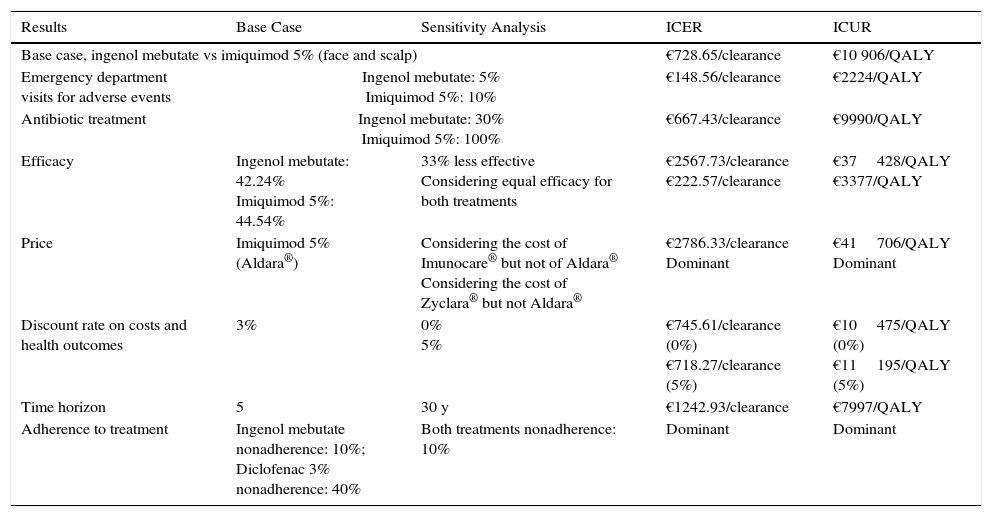
The deterministic sensitivity analyses confirmed the model's consistency in the 3 scenarios studied. Ingenol mebutate emerged as a dominant option vs diclofenac 3% in all the sensitivity analyses regardless of body site being treated (Table 5 and Appendix BS1, online supplementary material). Our findings also show that treatment of face and scalp AK with ingenol mebutate could be considered a cost-effective strategy in comparison with imiquimod 5%, given that it proved efficient in 80% of the sensitivity analysis (applying an efficiency threshold of €30000/QALY17). The ICUR exceeded that efficiency threshold in 2 situations: when a 33% (vs 50%) decrease in efficacy was assumed to be associated with nonadherence and when the cost of generic imiquimod 5% was used rather than the price of the brand-name product (Table 6 and Appendix BS2, online supplementary material).
Deterministic Sensitivity Analysis Results for Ingenol Mebutate vs Diclofenac 3% (Face and Scalp and Trunk and Extremities).
| Face and Scalp | ||||
|---|---|---|---|---|
| Parameters | Base Case | Sensitivity Analysis | ICER | ICUR |
| Base case, ingenol mebutate vs diclofenac 3% (face and scalp) | Dominant | Dominant | ||
| Emergency department visits for adverse events | Ingenol mebutate: 5%; diclofenac 3%: 2% | Dominant | Dominant | |
| Antibiotic treatment | Ingenol mebutate: 30%; diclofenac 3%: 5% | Dominant | Dominant | |
| Efficacy | Ingenol mebutate: 42.24%; diclofenac 3%: 24.64% | 33% less effective | Dominant | Dominant |
| Discount rate on costs and health outcomes | 3% | 0% 5% | Dominant | Dominant |
| Time horizon | 5 y | 30 y | Dominant | Dominant |
| Adherence to treatment | Ingenol mebutate nonadherence: 10%; Diclofenac 3% nonadherence: 40% | Both treatments nonadherence: 10% | Dominant | Dominant |
| Trunk and Extremities | ||||
|---|---|---|---|---|
| Results | Base Case | Sensitivity Analysis | ICER | ICUR |
| Base case, ingenol mebutate vs diclofenac 3% (trunk and extremities) | Dominant | Dominant | ||
| Emergency department visits for adverse events | Ingenol mebutate: 5%; diclofenac 3%: 5% | Dominant | Dominant | |
| Antibiotic treatment | Ingenol mebutate: 30%; diclofenac 3%: 5% | Dominant | Dominant | |
| Efficacy | Ingenol mebutate: 34.07%; diclofenac 3%: 25.07% | 33% less effective | Dominant | Dominant |
| Discount rate on costs and health outcomes | 3% | 0% 5% | Dominant | Dominant |
| Time horizon | 5 y | 30 y | Dominant | Dominant |
| Adherence to treatment | Ingenol mebutate nonadherence: 10%; Diclofenac 3% nonadherence: 40% | Both treatments nonadherence: 10% | Dominant | Dominant |
Abbreviations: ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio.
Deterministic Sensitivity Analysis Results for Ingenol Mebutate vs Imiquimod 5% (Face and Scalp).
| Results | Base Case | Sensitivity Analysis | ICER | ICUR |
|---|---|---|---|---|
| Base case, ingenol mebutate vs imiquimod 5% (face and scalp) | €728.65/clearance | €10 906/QALY | ||
| Emergency department visits for adverse events | Ingenol mebutate: 5% Imiquimod 5%: 10% | €148.56/clearance | €2224/QALY | |
| Antibiotic treatment | Ingenol mebutate: 30% Imiquimod 5%: 100% | €667.43/clearance | €9990/QALY | |
| Efficacy | Ingenol mebutate: 42.24% Imiquimod 5%: 44.54% | 33% less effective Considering equal efficacy for both treatments | €2567.73/clearance €222.57/clearance | €37428/QALY €3377/QALY |
| Price | Imiquimod 5% (Aldara®) | Considering the cost of Imunocare® but not of Aldara® Considering the cost of Zyclara® but not Aldara® | €2786.33/clearance Dominant | €41706/QALY Dominant |
| Discount rate on costs and health outcomes | 3% | 0% 5% | €745.61/clearance (0%) €718.27/clearance (5%) | €10475/QALY (0%) €11195/QALY (5%) |
| Time horizon | 5 | 30 y | €1242.93/clearance | €7997/QALY |
| Adherence to treatment | Ingenol mebutate nonadherence: 10%; Diclofenac 3% nonadherence: 40% | Both treatments nonadherence: 10% | Dominant | Dominant |
Abbreviations: ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio; QALY, quality-adjusted life-years.
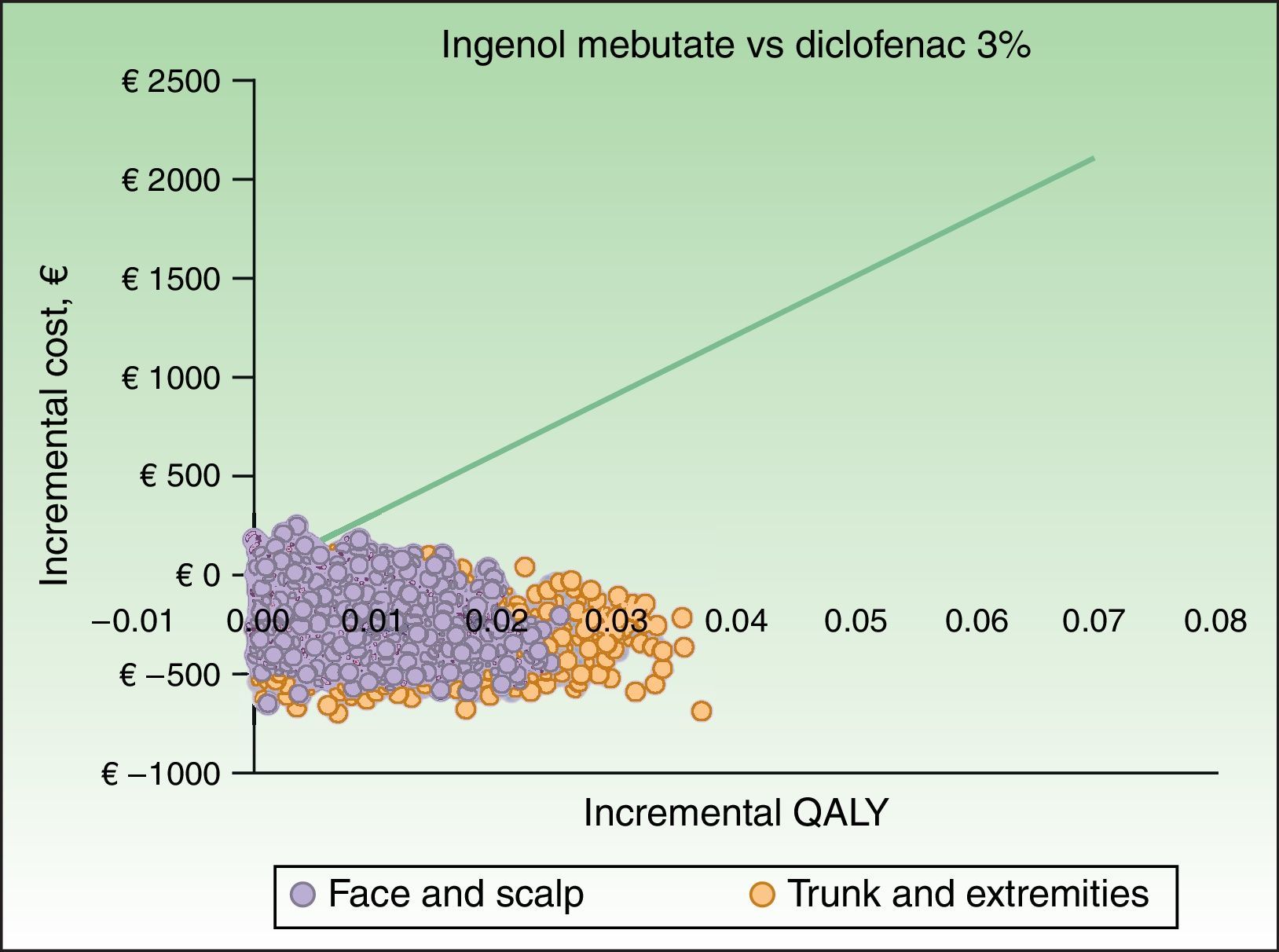
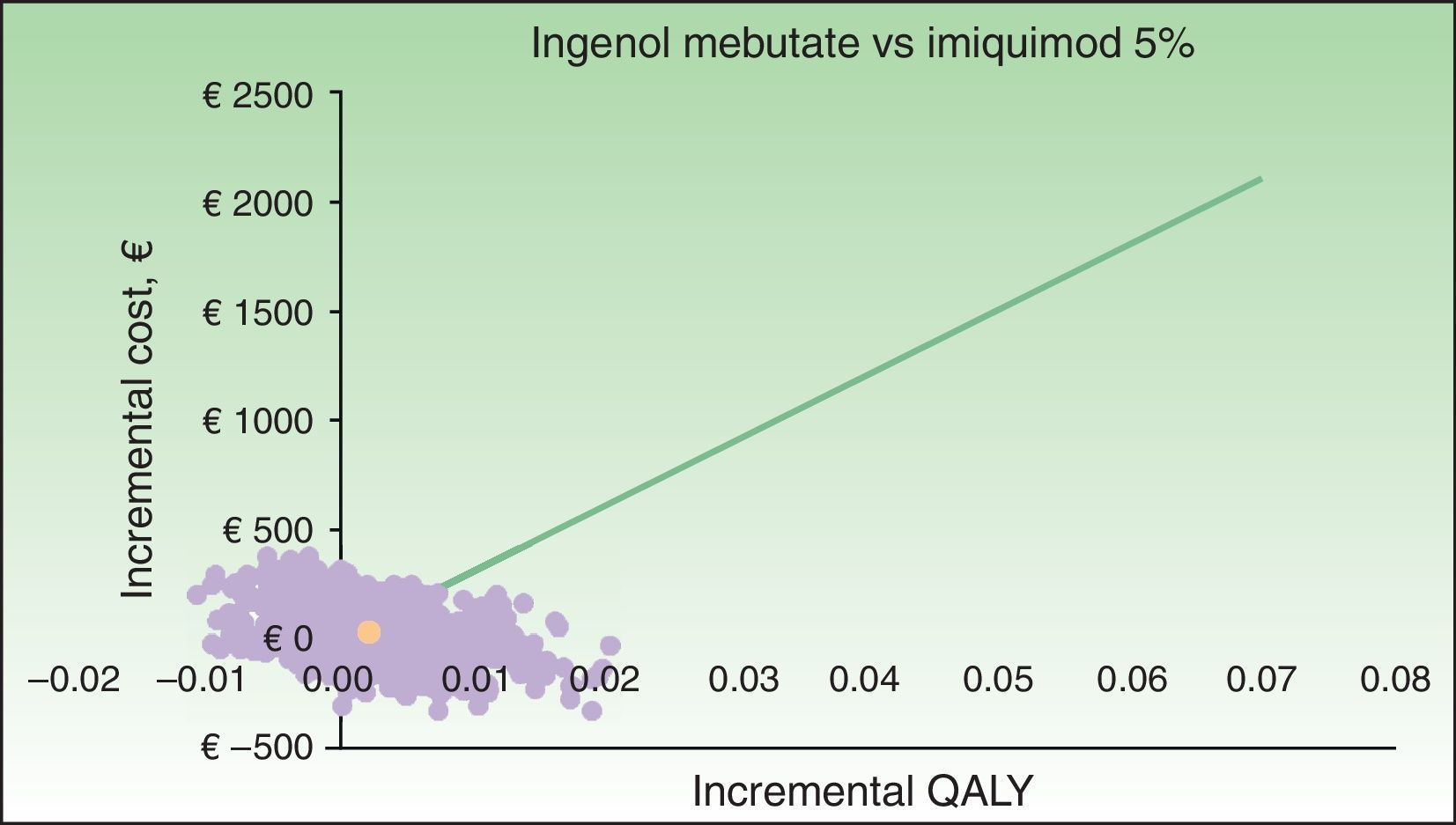
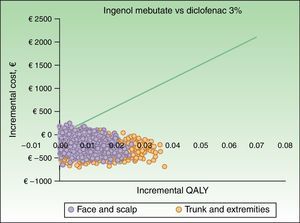
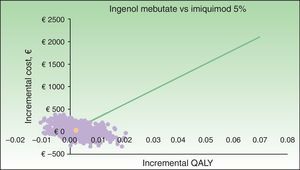
The results of the probabilistic sensitivity analysis are shown grafically using a scatterplot, where each point represents an ICUR for one of the 1000 simulations performed (Figs. 2 and 3). Thus, when ingenol mebutate was compared with diclofenac 3%, 96% of the simulations of face and scalp and 91% of trunk and extremity treatments had ICURs under the €30000/QALY threshold17 (Fig. 2). In the scenario comparing ingenol mebutate to imiquimod 5%, 80% of the simulations were cost-effective (Fig. 3).
The high population prevalence of AK has led to a substantial economic burden for health systems. It is therefore important to find more effective treatment alternatives that can reduce the duration of disease and frequency of recurrences, thereby lowering treatment-associated costs and contributing to NHS sustainability.
The patient profile in this disease, which appears in persons of advanced age, makes it difficult to choose the best therapeutic alternative. An indicator like the ICER, which brings together information on costs and health outcomes in addition to treatment durations, can be very helpful horizons, given that shorter treatments increase patient adherence25 and play a direct role in increasing efficacy. Even though the 3 treatments we evaluated (ingenol mebutate, diclofenac 3%, and imiquimod 5%) give the expected skin responses, quality of life improvements are evident in AK patients treated with ingenol mebutate because they are not affected by side effects that diminish that quality or satisfaction.26
A strength of this study is that it is the only cost-effectiveness analysis of AK treatments used currently in Spain (with the exception of photodynamic therapy) that has been validated by a panel of experts. One other pharmacoeconomic analysis of AK treatment in Spain appeared recently,27 but the alternatives considered were not those of the present analysis. Two studies done in Scotland28 and Finland29 compared ingenol mebutate to diclofenac 3% and imiquimod 5%. Both studies looked at treatment from their NHS perspectives and both concluded that ingenol mebutate was a cost-effective choice22 (higher costs and better health outcomes) and dominant23 (lower costs and better health outcomes) vs diclofenac 3%, and also cost-effective vs imiquimod 5%.23
The present study also reviewed data from the various clinical trials of the treatments studied, grouping them according to the body sites where they are applied. This approach meant that we assessed efficacy as conservatively as possible in a situation requiring indirect comparisons between treatments.
The consistency of the model was supported by sensitivity analyses applying modifications of parameters that might have compromised the analysis: in spite of the numerous permutations considered, the results still showed that ingenol mebutate is a dominant treatment option in comparison with diclofenac 3% (i.e., ingenol mebutate offers greater efficacy at a lower incremental cost) and cost-effective in comparison with imiquimod 5%. These results were consistent across all the analyses except the 2 mentioned previously. The parameter modifications that had the greatest impact on sensitivity analyses were the decrease in assumed level of efficacy in nonadherent patients and the lower cost of using the generic formulation of imiquimod. With those modifications the ICUR exceeded the threshold of €30000/QALY17 (and €2500/clearance).
This study has certain limitations that should be taken into account when interpreting the results. Among them is the theoretical nature inherent to any modeling study, as projections may not faithfully represent the reality of routine clinical practice. We chose to use a Markov model that allowed us to incorporate repeated events such as the possibility of recurrent AK episodes in a single patient. However, other approaches to economic evaluation of interventions in this disease have used decision trees, which can be easier to interpret intuitively. The possibility of transitioning to a state of death may seem farfetched in a disease that is not associated with high mortality, but it was considered necessary for estimating QALY (providing a survival endpoint for the cost-utility threshold). In any case, the model and the determination of the states of health contemplated were constrained by the availability of clinical data for carrying out the simulation.
Another possible limitation concerns the method used to designate efficacy variables. Given the absence of clinical trials directly comparing the treatment alternatives we studied, we used indirect methods to infer levels of efficacy. Indirect comparisons, can be more biased than direct ones and may overestimate an intervention's efficacy.30 However, the use of indirect treatment comparisons has become a standard, widely recognized approach to use when direct evidence from randomized trials is unavailable or inadequate. Indirect comparisons can provide useful, complementary information about the relative efficacy of interventions if and only if they have high internal and external validity and the clinical trials are similar.31 They also provide useful evidence for judiciously selecting the best treatment.32 Nevertheless, the comparisons made in this study should ideally be repeated using data from direct treatment comparisons.
Another limitation stems from our use of data from studies performed in other countries; this decision was necessary because this type of study has not been done in Spain. The utility values used in the model were derived from a study done in the United Kingdom.13 They were validated by our panel of experts, who considered these values to be representative of the population of patients with AK in Spain. They are also those defined for the EuroQol 5-dimensions questionnaire and do not vary across different European countries.33
In spite of these limitations, this economic evaluation can facilitate prescribing physicians’ decisions given that ingenol mebutate is a dominant therapy with lower costs for the NHS and leads to more effective health outcomes for patients in comparison with diclofenac 3%. In addition, if €30000/QALY is accepted as a valid threshold of efficiency,17 ingenol mebutate can be considered a cost-effective alternative compared to imiquimod 5% for treating AK lesions on the face and scalp in Spain.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestI. Elías, N. Ortega-Joaquín, and I. Oyagüez, who are employed by PORIB, a consultancy firm specializing in cost-effectiveness analyses of health care technologies, received an unconditional grant from LEO-Pharma to carry out the analyses for this paper.
A. Miranda, E. Mosquera, and C. Gibbons are employees of LEO-Pharma.
P. de la Cueva, L. J. del Pozo, D. Moreno-Ramírez, A. Boada, and M. Aguilar participated as expert consultants to validate the parameters used for this analysis; the unconditional grant they received from LEO-Pharma for their participation at no time influenced their opinions or the conclusions they drew.
Please cite this article as: Elías I, Ortega-Joaquín N, de la Cueva P, del Pozo LJ, Moreno-Ramírez D, Boada A, et al. Análisis de coste-efectividad y coste-utilidad de ingenol mebutato versus diclofenaco 3% e imiquimod 5% en el tratamiento de la queratosis actínica en España. Actas Dermosifiliogr. 2016;107:498–508.