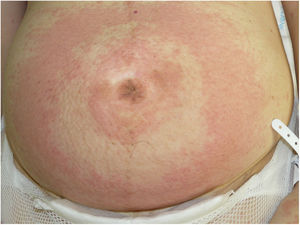
Gestational pemphigoid (PG) is a rare autoimmune bullous disease that affects women during pregnancy and is characterized by the appearance of plaques, erythematous papules, and/or highly pruritic blisters that usually first appear in the periumbilical area.1
PG occurs in the second or third trimester of pregnancy or during the puerperal period, and can cause fetal complications such as low weight, malformations, and preterm birth.1,2
PG should be differentiated from other dermatoses of pregnancy. Biopsy for conventional histology is of little use in this regard. Diagnosis is established using direct or indirect immunofluorescence or antigen detection techniques (mainly enzyme-linked immunosorbent assay [ELISA], although immunoblot and immunoprecipitation can also be used).1
Treatment selection is complicated owing to the limited number of safety studies performed in pregnant women. Most systemic corticosteroids are considered safe, even though their potential adverse effects include teratogenic effects (orofacial anomalies), risk of miscarriage, and antepartum fetal death.3,4
Although the majority of cases respond adequately to systemic corticosteroids, in some cases adjuvant therapies are required to attenuate clinical signs and reduce the corticosteroid dose and treatment duration.
We present 4 cases of PG that were refractory to corticosteroids and required treatment with intravenous immunoglobulin (IVIG) therapy.
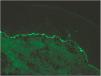
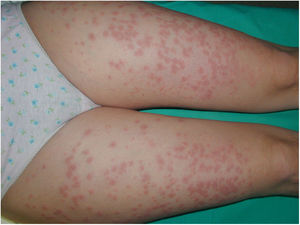
Case 1A 44-year-old woman who was pregnant with twins presented with pruritic erythematous plaques that appeared on the abdomen and both lower extremities at week 16 of gestation and subsequently spread to the forearms and trunk. Biopsy and direct immunofluorescence (DIF) confirmed the diagnosis of PG (Fig. 1).
The patient began treatment with oral prednisone (20 mg/d, subsequently increasing to 30 mg/d). In the absence of any improvement, the multidisciplinary team opted for monthly IVIG cycles (1 g/kg/d on 2 consecutive days), following our center’s protocol.
A marked improvement in the patient’s lesions was observed after the first dose, and therefore the dose of prednisone was progressively reduced to 10 mg/day. The patient received 4 treatment cycles, and achieved complete remission at week 34 of gestation. A Caesarean section was performed for breech presentation of the first twin at week 36.
The puerperal period passed without incident, and corticosteroid therapy was withdrawn 1 month after birth (Fig. 2).
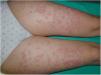
Case 2A 42-year-old woman presented with highly pruritic bullous lesions on the abdomen and lower extremities that appeared at week 24 of gestation. DIF of a biopsy sample confirmed a diagnosis of PG. The patient began treatment with prednisone (30 mg/d). The lesions continued to evolve, and at week 30 of gestation it was decided to begin treatment with monthly IVIG cycles. A clear improvement was observed after the first treatment cycle, and the prednisone dose was reduced to 20 mg/day. At week 35 of gestation, a Caesarean section was performed due to failed induction after premature rupture of membranes. The lesions disappeared after childbirth, and treatment was discontinued without incident (Fig. 3).
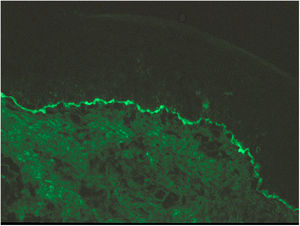
Case 3A 32-year-old woman presented at week 28 of gestation with a rash consisting of erythematous vesicles and plaques on the abdomen. Histological and DIF findings confirmed a diagnosis of PG. The patient began treatment with prednisone (30 mg/d). In the absence of any improvement, she subsequently began IVIG therapy. Clinical improvement was observed after the first IVIG cycle at week 30. Due to sudden weight gain, the patient’s corticosteroid dose was progressively decreased to 10 mg/day. A second cycle of IVIG was administered at week 34. Due to the appearance of gestational thrombocytopenia, the prednisone dose was increased to 20 mg/day. Childbirth occurred spontaneously at week 35 of gestation, without incident. The lesions reappeared 3 weeks postpartum, and therefore corticosteroid therapy was continued for 2 more months.
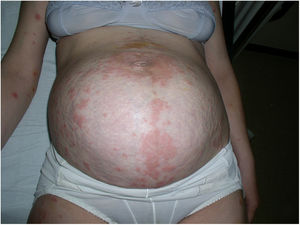
Case 4A 40-year-old woman presented at week 31 of gestation with erythematous and edematous plaques and vesicles distributed predominantly on the abdomen, although the rest of the trunk and the extremities were also affected. PG was diagnosed based on the results of DIF of a skin biopsy sample. Treatment with oral prednisone (30 mg/d) provided good control during pregnancy. A Caesarean section was performed at week 37 of gestation after failure of induction due to gestational diabetes. The postoperative period was uneventful. Exacerbation of the lesions during the first month of the puerperal period necessitated an increase in the corticosteroid dose to 60 mg/day. In the absence of any clear improvement, it was decided to begin IVIG treatment. Little improvement was observed after the first IVIG cycle. Another 2 cycles were scheduled, the corticosteroid dose was decreased to 40 mg/day, and the patient began treatment with methotrexate (15 mg/wk). After the third IVIG cycle it was possible to taper the dose of corticosteroids and methotrexate until complete resolution of the lesions 12 months later (Fig. 4).
IVIG therapy resulted in no adverse effects in any of the 4 cases presented here.
Oral corticosteroids and antihistamines are considered first-line treatment for PG.5 However, when these therapies are insufficient, the therapeutic range is reduced due to adverse effects and the potential embryotoxic effects of other immunosuppressive medications.
In such cases, IVIG therapy appears to be a safe option, and has proven effective for the treatment of other diseases with similar etiologies, including pemphigus vulgaris and epidermolysis bullosa.6
There are very few published cases of refractory PG treated with IVIG.2,3,7–10 To our knowledge, this is the most extensive series published to date.
Conflicts of InterestThe authors declare that they have no conflicts of interest
Please cite this article as: Boria F, Maseda R, Albízuri F, De la Calle M. Tratamiento con inmunoglobulinas intravenosas del penfigoide gestacional refractario al tratamiento convencional. Actas Dermosifiliogr. 2021;112:83–85.