Panitumumab is a monoclonal antibody that inhibits epidermal growth factor (EGF) by binding to its extracellular domain.1 It is approved for the treatment of metastatic colorectal carcinoma in patients who express EGF receptor and wild-type KRAS. Adverse cutaneous effects can occur in up to 90% of patients.1 We present a case of panitumumab-associated inflammation of subclinical actinic keratosis, an adverse effect not described in the literature to date.
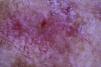
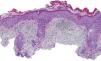
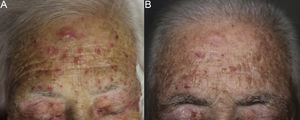
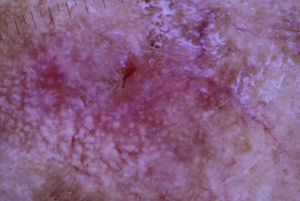
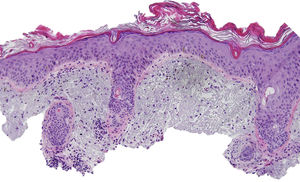
The patient was an 80-year-old woman who in 2007 had been diagnosed with stage IV KRAS wild-type adenocarcinoma with metastasis in the liver, lung, mediastinum, and peritoneum. After progression during several lines of chemotherapy she began intravenous panitumumab monotherapy every 3 weeks. Two months after beginning panitumumab treatment she attended the dermatology department with erythematous-desquamative lesions on the face that were rough to the touch and in some cases erosive (Fig. 1). She had no history of skin problems and was taking no other medication. Dermatoscopy of the lesions showed an erythematous pseudoreticulum with a distinctive strawberry pattern (Fig. 2). Biopsy of one of the lesions revealed the presence of atypical keratinocytes, which were largely confined to the basal layer of the epidermis. Marked solar elastosis, predominantly perifollicular lymphocytic infiltrate, and scattered melanophages were evident in the dermis (Fig. 3). The patient was diagnosed with grade 1 actinic keratosis with inflammation,2,3 for which she was treated for 2 weeks with a topical corticosteroid and antibiotics. She received a total of 8 cycles of panitumumab therapy. Five months later the lesions reappeared, leaving a residual pink macula (Fig. 1B).
Histological image showing atypical keratinocytes in the basal layer of the epidermis and hyperkeratosis in the follicular infundibulum. Solar elastosis, melanophages, and predominantly perifollicular lymphocytic infiltrate are evident in the dermis (hematoxylin and eosin, original magnification×10).
Cutaneous effects of panitumumab include alterations of the hair and nails, mucositis, photosensitivity, xerosis, fissures, and papulopustular eruptions, and may necessitate dose reduction.4
Inflammation of actinic keratosis is common in patients treated with imiquimod, ingenol mebutate, or topical 5-fluorouracil, and its intensity appears to be related to the degree of clinical response.5 Subclinical actinic keratosis in the field of cancerization is also frequently observed following treatment with these agents,5 and following systemic monotherapy with a variety of chemotherapeutic agents including fluorouracil, capecitabine, doxorubicin, deoxycoformycin, cisplatin, docetaxel, and fludarabine. Inflammation of actinic keratosis has also been reported in patients treated with combination therapies (dactinomycin, dacarbazine, and vincristine, and doxorubicin, cytarabine, and 6-thioguanine),6 and with new target therapies, including sorafenib, sunitinib, and erlotinib.7–9 However, we have found no reports linking panitumumab therapy to inflammation of actinic keratosis.
The pathogenesis of this reaction remains unclear. Inflammation of actinic keratosis in patients treated with classical chemotherapeutic agents may reflect a direct cytotoxic effect on atypical keratinocytes or radiation recall in the field of cancerization.8 In the case of sunitinib, an antiangiogenic effect on atypical keratinocytes is suspected.7
EGF receptor function is dysregulated in actinic keratosis and squamous cell carcinoma.10 It is therefore possible that panitumumab-induced inhibition of this receptor triggered the inflammation of actinic keratosis in our patient. The preferential accumulation of the drug in the follicular infundibulum could favor hyperkeratinization and secondary inflammation of subclinical actinic keratosis, as previously proposed for erlotinib.9 It remains unknown whether this cutaneous reaction is associated with a better treatment response, as described for erlotinib.9
In summary, we present the first described case of panitumumab-associated inflammation of subclinical actinic keratosis. While this adverse effect has been previously described for some classic chemotherapeutic drugs and new target therapies, the underlying mechanism remains unknown.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Escudero-Góngora MM, del Pozo-Hernando LJ, Corral-Magaña O, Antón E. Inflamación de queratosis actínicas durante el tratamiento con panitumumab. Actas Dermosifiliogr. 2018;109:749–751.