Kathon CG is a preservative that contains methylchloroisothiazolinone (MCLI) and methylisothiazolinone (MI) in a 3 to 1 ratio. It is used in cosmetics as well as in industrial and domestic cleaning products. An increase in sensitization to MCLI/MI has recently been reported, probably due to an increase in sensitization to the MI component.1
We have performed a retrospective analysis of the data from patients who underwent patch testing in our hospital to discover whether this situation has also arisen in our setting. The sample of patients sensitized to MCLI/MI includes from January 1, 2005, to July 31, 2013, and the sample of those sensitized to MI includes from January 1, 2012, to July 31, 2013. The χ2 test (Epidat 4.0®, Servicio Gallego de Salud) was used to determine whether significant differences existed between the groups studied. Sensitization to MCLI/MI was diagnosed using the True Test (Smartpractice DENMARK ApS, Hillerød, Denmark) and all patients evaluated during the study period underwent patch testing for MI at a concentration of 0.05%in water (alergEAZE, MartiTor, Barcelona) and at 0.2%in water in those patients patch tested with the cosmetics series (Chemotecnique, Vellinge, Sweden).
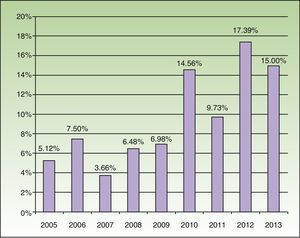
Patch testing for MCLI/MI was performed in 863 patients, of whom 85 (9.85%) were sensitized. Analysis of the frequency of sensitization by years revealed a marked increase starting in the year 2010 (Fig. 1). When the study period was divided at the 2010 time point, we observed an increase in the frequency of sensitization from 5.97% to 14.11% (P<.05, χ2 test). Comparison of the MOAHLFA indices of the sensitized patients in these 2 periods showed no significant differences; this would suggest there had been no epidemiological changes that might justify this increase in frequency. However, evaluation of the cause of the eczema showed a statistically significant increase in sensitization related to the use of cosmetic products in the second period (P<.05, χ2 test) (Table 1).
MOAHLFA Index of the Study Population and of the Subgroup of Patients Sensitized to MCLI/MI.
| Study Population(863 Patients) | Positive to MCLI/MI(85 Patients) | Positive to MCLI/MI 2005-2009(27 of 457 Patients) | Positive to MCLI/MI 2010 to June 2013(58 of 411 Patients) | |
| M (male) | 27.35% | 31.76% | 40.74% | 27.59% |
| O (occupational) | 10.20% | 11.76% | 18.52% | 8.62% |
| A (atopy) | 16.34% | 25.88% | 37.04% | 20.69% |
| H (hand) | 32.91% | 42.35% | 40.74% | 41.38% |
| L (leg) | 32.79% | 35.29% | 44.44% | 31.03% |
| F (face) | 33.95% | 41.18% | 44.44% | 39.66% |
| A (age>40) | 60.14% | 62.35% | 59.26% | 60.34% |
| Cosmetic origina | 16.22% | 43.53% | 25.93% | 51.72% |
MI was studied in 195 patients, of whom 8.2% (16 patients) were sensitized. It should be noted that 5 of the 16 patients patch tested with MI at both concentrations (0.05% and 0.2%) were negative at the lower concentration. All the patients sensitized to MI were positive with MCLI/MI, and 50% of those sensitized to MCLI/MI were positive with MI.
Since its introduction in the eighties, MCLI/MI has been found to be a powerful sensitizer, and the maximum permissible concentration in cosmetic products has therefore been reduced to 15ppm. Despite this regulation, the frequency of sensitization prior to 2008 was stable in Europe at levels between 1% and 4%,2 and in Spain at figures between 3% and 4%.3,4 However, since that date, the frequency of sensitization in Europe has doubled1,5,6 and, in 2012, reached 8% in Spain.7 Our data show that sensitization levels were already very high and that they have doubled in the past 3 years.
In 2005, MI was approved for use in cosmetic products at a maximum concentration of 100ppm, but its concentration has not been regulated in industrial products. It is estimated that the prevalence of sensitization to MI in Europe was around 1.5% prior to 2010, and it has increased in parallel with MCLI/MI sensitization in recent years. This finding allows us to state that this new sensitization “epidemic” to MCLI/MI is due in large part to the increase in primary sensitization to MI.8 In our population, both the high frequency of sensitization to MI (8.2% in the period evaluated), and the close parallel with the results of patients positive to MCLI/MI would favor this conclusion. The results presented also support the current recommendation to perform patch testing for MI at a concentration of 2000ppm, as there is a loss of sensitivity when the test is performed with a concentration of 500ppm. According to the main European studies, patch testing only the MCLI/MI mixture, without independently testing MI, would fail to detect between 30% and 60% of patients sensitized to MI8; however, all our patients sensitized to MI were positive to MCLI/MI. Our patients were patch tested with MCLI/MI in the TrueTest and not with this allergen diluted in water. The technique is thus different and the data are not therefore wholly comparable.
In conclusion, there is a high frequency of sensitization to MCLI/MI in Spain, and it has increased significantly since 2010. This requires regulation sooner rather than later to control the use of MI both in cosmetics and in industrial products.
Please cite this article as: Liuti F, Hernández Hernández Z, Borrego Hernando L. Aumento de sensibilización al Kathon CG® (clorometilisotiazolinona/metilisotiazolinona) en el área sur de Gran Canaria. Actas Dermosifiliogr. 2014;105:882–883.