Dermatopathology includes a long list of disorders, some of which have very similar histopathology. Immunohistochemistry is an important auxiliary tool for diagnosis and differential diagnosis, and for predicting the outcome of many skin tumors. It is also the main technique for determining the origin of a tissue or the differentiation of neoplastic cells. In many cases, immunohistochemistry provides a more accurate diagnosis of the different processes that infiltrate the skin. This review examines the role of immunohistochemistry in studying the differentiation and biological behavior of the majority of tumors that can involve the skin. We review immunoperoxidase techniques, discuss the utility of the most commonly used antibodies, and highlight a number of diagnostic problems in which immunohistochemistry may be very useful. In each case, the goal is to reach a specific and definitive diagnosis. In the second part of our review, we examine the most useful and specific antibodies in the study of skin infections and of epithelial, muscular, lymphatic and hematologic, neural, neuroendocrine, and melanocytic neoplasms that affect the skin. Finally, we include a brief review of the immunohistochemical profile of skin metastases of malignant visceral tumors.
La dermatopatología incluye una larga lista de entidades, algunas con una histopatología muy similar. La immunohistoquímica representa una importante herramienta de ayuda en el diagnóstico, diagnóstico diferencial y pronóstico de muchas de las neoplasias cutáneas. La inmunohistoquímica es también la mejor técnica para determinar el origen de un tejido o la diferenciación de las células neoplásicas. En muchos casos, la inmunohistoquímica permite un diagnóstico más preciso de los distintos procesos infiltrando la piel. Este artículo revisa el papel de la inmunohistoquímica en el estudio de la diferenciación y el comportamiento biológico de la mayoría de las neoplasias que pueden afectar a la piel. Se revisan las técnicas de inmunoperoxidasa, se discute la utilidad de los anticuerpos utilizados con mayor frecuencia y se presentan una serie de problemas diagnósticos en los que la immunohistoquímica puede resultar muy útil. En cada caso, la finalidad es llegar a un diagnóstico concreto y definitivo. En esta segunda parte de nuestra revisión analizan los anticuerpos más útiles y específicos en el estudio de las infecciones cutáneas, así como de las neoplasias epiteliales, musculares, vasculares, linfo-hematológicas, neurales, neuroendocrinas y melanocíticas afectando a la piel. Al final, se incluye una breve revisión del perfil inmunohistioquímico de las metástasis cutáneas de neoplasias malignas viscerales.
Only a small group of microbial antibodies are of actual use in dermatopathology, as other diagnostic methods, such as culture or polymerase chain reaction (PCR), have a superior diagnostic yield. Furthermore, immunohistochemical detection of microorganisms or antigens of microbial origin in skin infections requires a strong index of suspicion.1
Immunostaining with anti-cytomegalovirus antibody gives a nuclear staining pattern in early-stage infections and a nuclear and cytoplasmic pattern in late-stage infections.
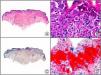
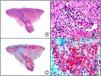
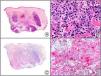
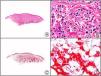
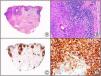
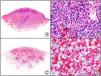
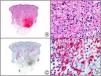
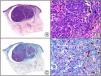
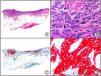
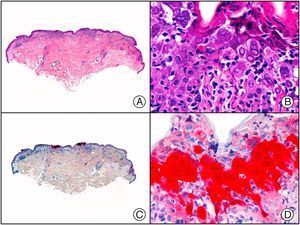
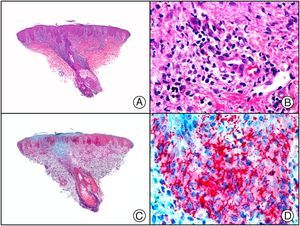
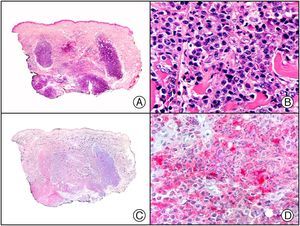
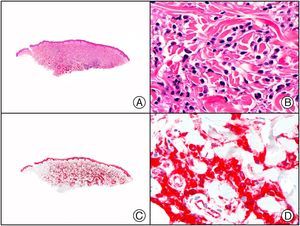
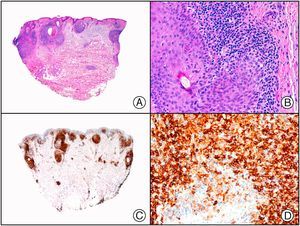
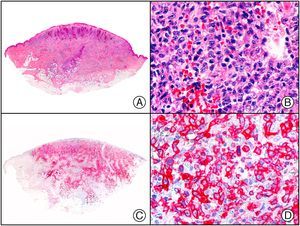
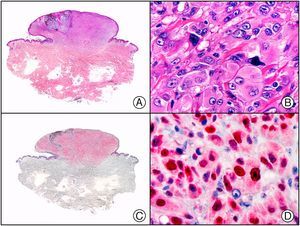
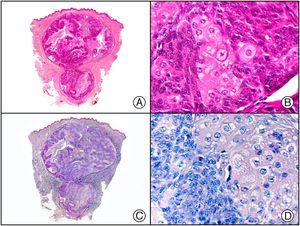
There are also commercial antibodies that can identify gene products from herpes simplex virus (HSV) types 1 and 2 (Fig. 1) and varicella-zoster virus (VZV) in skin infections. In all cases, the cells exhibit both nuclear and cytoplasmic staining. Nonetheless, the intensity of staining varies between cells depending on the type of infection. In infections by HSV-1 and HSV-2, for example, epidermal keratinocytes show the strongest staining, while in infections due to VZV, the strongest staining is seen in the cells of the outer root sheath of the hair follicle and in sebocytes of the sebaceous gland. Epidermal keratinocytes, on the contrary, tend to be negative for VZV, at least in the early stages of infection.2
HSV (herpes simplex virus infection). A, Low-power magnification. B, Characteristic cytopathic effect of HSV, showing nuclei of keratinocytes with peripheral margination of chromatin. C, The same specimen studied immunohistochemically with anti-HSV-1 antibody. D, Positive staining with anti-HSV-1 antibody in the same cells showing the cytopathic effect of HSV.
Immunostaining with EPV-LMB (Epstein-Barr virus–encoded latent membrane protein) yields a cytoplasmic pattern. This marker is useful in the diagnosis of posttransplant lymphoproliferative disorder, Hodgkin lymphoma, and other lymphomas. It is also positive in 25% to 50% of nasopharyngeal carcinomas. In dermatopathology, EBV-LMB is also useful for demonstrating the presence of EBV gene products in hairy leukoplakia lesions in patients with acquired immunodeficiency syndrome.3,4
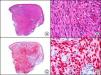
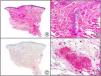
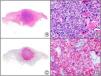
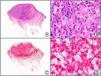
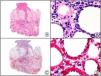
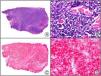
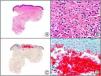
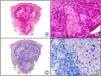
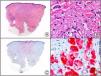
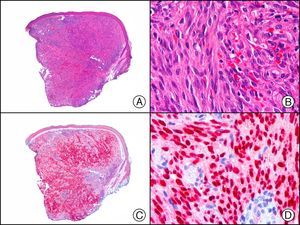
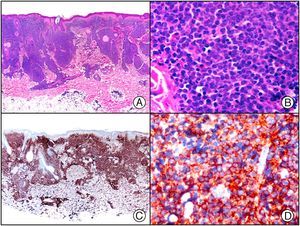
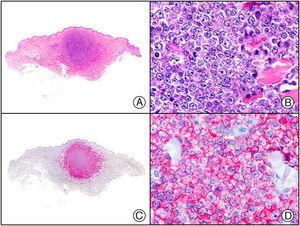
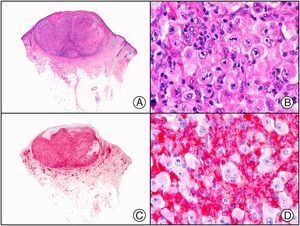
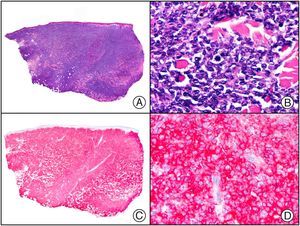
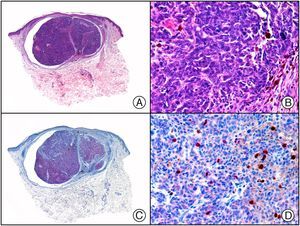
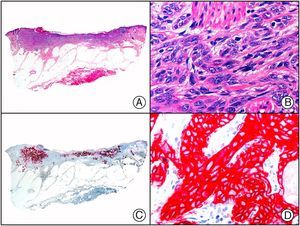
Staining with anti-HHV-8 (human herpes virus 8) antibody yields a nuclear pattern. HHV-8 infects different types of cells, in which it is generally expressed in the latent form as an episomal nuclear structure. The antibody recognizes latent nuclear antigen of HHV-8 involved in the pathogenesis of several tumors, including Kaposi sarcoma (Fig. 2), systemic Castleman disease, and primary effusion lymphoma, and is a very useful diagnostic tool in these settings. It is also of value in differentiating these entities from mimickers.5,6
Nodular-stage Kaposi sarcoma. A, Low-power magnification. B, Detail of spindle-cell morphology of neoplastic cells intermingled with several red blood cells. C, The same specimen studied immunohistochemically with anti-HHV (human herpes virus) 8 antibody. D, HHV-8 positivity in the nuclei of most neoplastic cells.
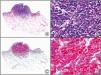
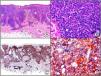
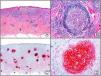
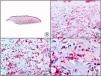
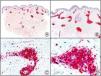
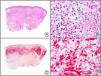
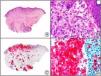
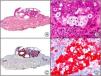
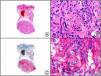
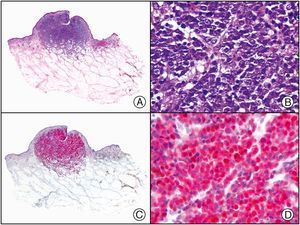
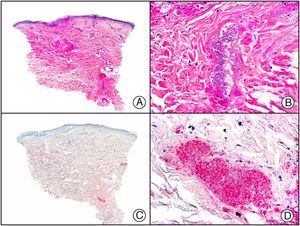
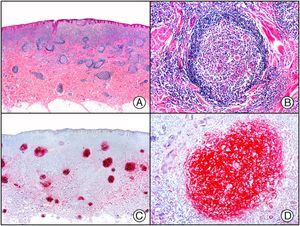
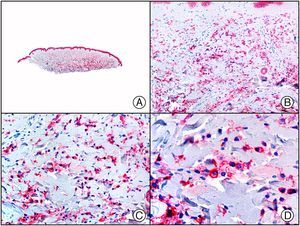
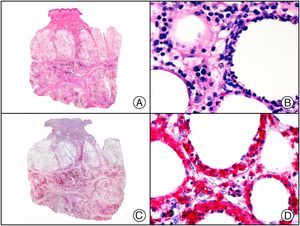
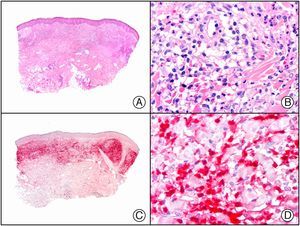
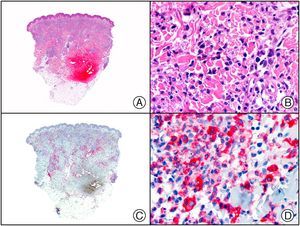
The clonal integration of a new human polyomavirus was recently demonstrated in the nucleus of Merkel cell carcinoma cells, with viral DNA detected in tumor cells by both PCR and immunohistochemistry7 (Fig. 3). This polyomavirus, however, is not exclusive to Merkel cell carcinoma, as it has also been observed in certain squamous cell carcinomas in immunosuppressed patients. It does, however, appear to play a key role in the histogenesis of Merkel cell carcinoma.Human parvovirus B19 can be detected immunohistochemically in the endothelium of congested capillaries in the papillary dermis in skin lesions in papular-purpuric gloves and socks syndrome (Fig. 4) and infectious erythema.8–10
Papular-purpuric gloves and socks syndrome. A, Low-power magnification. B, Lymphocytic infiltrate and extravasated red blood cells surrounding the postcapillary venules in the superficial dermis. C, The same sample studied immunohistochemically with anti-PVB (human parvovirus) 19 antibody. D, Cytoplasmic expression of PVP-19 in endothelial cells.
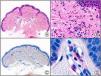
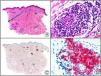
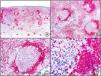
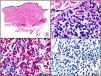
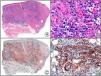
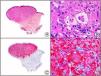
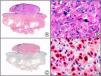
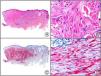
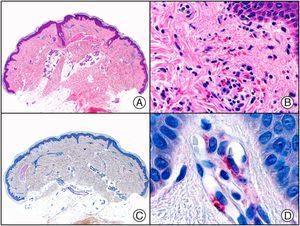
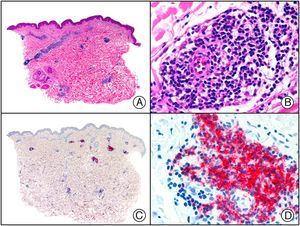
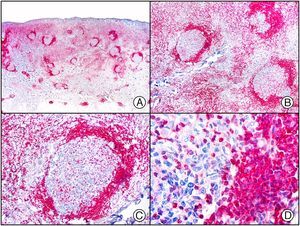
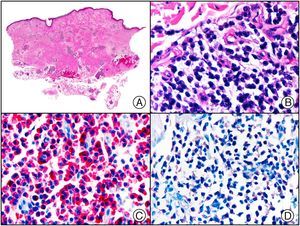
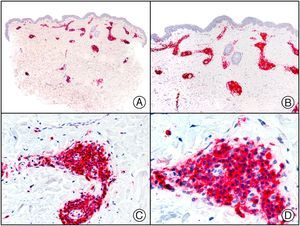
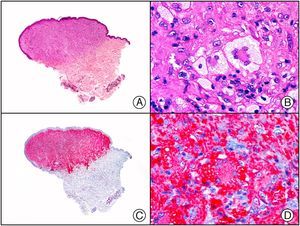
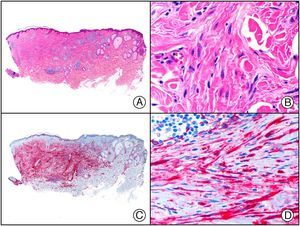
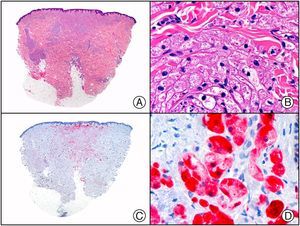
A new commercial polyclonal antitreponemal antibody recognizes Treponema pallidum, the bacteria responsible for syphilis, in formalin-fixed and even paraffin-embedded tissue, thus offering greater sensitivity and specificity than traditional silver impregnation methods, such as Steiner or Warthin-Starry stains.1 Different staining patterns have been observed in skin and mucosal lesions in primary and secondary syphilis. In primary syphilitic sores (chancres), there are more treponemes at the interface between the skin epithelium and the underlying dermis, and also around blood vessels in the papillary dermis (skin lesions) and the chorion (mucosal lesions).11 In secondary syphilis, however, abundant treponemes are only seen at the interface between the skin or mucosal epithelium and the underlying dermis or chorion (Fig. 5). It should, however, be borne in mind that this new antibody is not specific to T pallidum, as it also identifies other spirochetes, raising the risk of false positives when syphilis is suspected in mucosal lesions.
Secondary syphilis. A, Low-power magnification. B, Prominent endothelial and perivascular infiltrate with some plasma cells. C, The same specimen studied immunohistochemically with anti-Treponema pallidum antibody. D, High-power magnification showing numerous spirochetes dotted through the epidermal epithelium and showing strong staining for anti-T pallidum antibody.
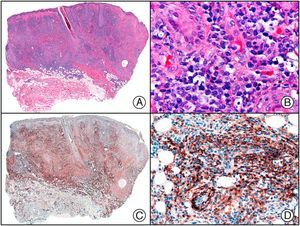
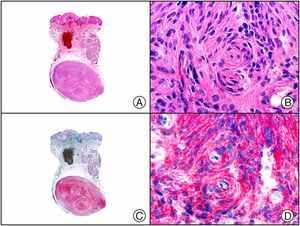
The anti-BCG polyclonal antibody (B124) has proven to be an extremely valuable screening tool for the detection of bacteria or fungi in skin biopsy specimens. It recognizes all mycobacteria and practically all gram-positive and gram-negative bacteria (Fig. 6), as well as the spores and hyphae of dermatophytes and other fungi. It does not recognize leishmaniasis or viral infections. It could perhaps be said that immunostaining with anti-BCG antibody produces similar results to those achieved with the combined use of Gram and periodic acid-Schiff staining.12
Ecthyma gangrenosum. A, Low-power magnification. B, High-power magnification showing aggregates of basophils in the lumen and the walls of the vessels, corresponding to numerous Pseudomona aeruginosa bacteria. C, The same specimen studied immunohistochemically with anti-BCG antibody. D, Detail of positive results obtained with anti-BCG antibody in the lumen and wall of a vessel in the deep dermis.
CD45 is a transmembrane glycoprotein with tyrosine phosphatase activity. It is also known as leukocyte common antigen and is expressed in myeloid leukocytes (Fig. 7) and in certain lymphoid cells, as well as in hematologic malignancies, such as Hodgkin and non-Hodgkin lymphoma, multiple myeloma, and even nonhematologic malignancies, such as histiocytic sarcoma or metastases from neuroendocrine tumors. The main isoforms of CD45 are CD45RO and CD45RA. CD45RA has the highest molecular weight of the two and is expressed in circulating naïve T cells recognized by anti-CD45RA monoclonal antibodies. CD45RO, by contrast, is expressed in circulating activated T cells and is associated with the acquisition of immune memory (memory T cells).13
CD20 is a B cell–specific marker. It is expressed in approximately 98% of all B-cell lymphomas and B-cell chronic lymphocytic leukemia (Fig. 8), in 50% of B-cell acute lymphoblastic leukemias (ALLs), and in just 10% of plasmablastic lymphomas, myelomas, and plasmacytomas. It is also positive in 20% of Reed-Sternberg cells in Hodgkin lymphoma. It is important to note that B-cell lymphomas treated with monoclonal anti-CD20 antibody (rituximab) may lose CD20 expression.14
CD79a is expressed before CD20 in the ontogenesis of B cells. It is, however, lost after CD20 during late differentiation stages, explaining why plasma cells are positive for CD79a and negative for CD20. CD79a stains both mature and immature B cells, and recognizes the majority of B-cell tumors, including B-cell ALL (precursor [pre-] B ALL), B-cell lymphoma treated with rituximab, and 50% of plasma cell neoplasms15 (Fig. 9).
B cell–specific transcription factors PAX-5 (paired box protein 5), BOB-1 (B-cell specific octamer binding protein 1), BLIMP-1 (B lymphocyte-induced maturation protein 1), OCT-2 (octamer-binding factor 2), MUM-1 (multiple myeloma oncogene 1), Bcl-6 (B-cell lymphoma 6), and CD10 can also be used as diagnostic immunohistochemical markers. PAX-5 is expressed in early development stages, while OCT-1 and BOB-1 are expressed in mature B cells; MUM-1 and BLIMP-1, by contrast, are associated with plasma cell differentiation. PAX-5 encodes a B cell–specific transcription factor expressed in pre-B cells and subsequently in B cells up to the moment they differentiate into plasma cells (in which it is downregulated and therefore not expressed). It yields a nuclear staining pattern in almost all B-cell lymphomas (including pre-B ALL), diffuse large B-cell lymphoma without plasmacytic differentiation (CD20-negative) and CD20-negative lymphomas following rituximab treatment. It is negative in tumors with plasma cell differentiation.15
MUM-1/IRF4 is a 50-kDa nuclear protein encoded by the MUM1 gene, which was originally identified in multiple myeloma. It is expressed exclusively in cells of lymphocytic and melanocytic origin. It is a marker of postfollicular B cells and activated cells, and is particularly useful for characterizing the histogenesis of large B-cell lymphomas. In addition to multiple myeloma (Fig. 10), it is expressed in diffuse large B-cell lymphoma and activated T cells. The study of MUM-1, CD10, and Bcl-6 expression helps to distinguish between 2 types of tumor with different prognoses: germinal center B-cell lymphomas and the much more aggressive activated B-cell lymphomas. MUM-1 and OCT-2 staining patterns have also been seen to be much stronger in primary cutaneous diffuse large B-cell lymphoma, leg type than in primary cutaneous follicle center lymphoma (FCL), 2 entities that are positive for Bcl-6.15
The proto-oncogene Bc16 encodes a 95-kDa protein expressed in normal tonsillar germinal center B cells and related lymphomas. It is involved in the 3q27 translocation associated with non-Hodgkin lymphoma, as well as in large B-cell lymphoma, Burkitt lymphoma, and nodular lymphocyte-predominant Hodgkin lymphoma and nodular sclerosis classical Hodgkin lymphoma. It is negative in primary cutaneous marginal zone B-cell lymphoma and positive in primary cutaneous FCL16 (Fig. 11).
Bcl-2 is an apoptosis-inhibiting protein. The proto-oncogene Bcl-2 is expressed in most T cells, but is absent from normal, activated B cells.17 It identifies marginal zone B-cell lymphomas and most diffuse large B-cell lymphomas, leg type, and is negative in the majority of cutaneous FCLs (Fig. 12). It can also be of use in identifying the origin of cutaneous follicular lymphoma as strong staining generally indicates spread of the tumor to the skin from the lymph nodes and therefore worse prognosis.16Table 1 summarizes the use of B cell–specific transcription factors in the diagnosis of cutaneous B-cell lymphomas.
The same specimen as that shown in Figure 11 studied immunohistochemically with Bcl-2 (B-cell lymphoma 2). Note that absence of Bcl-2 expression in the neoplastic B cells but the positive staining in a peripheral ring of reactive lymphocytes.
The expression of Bcl-1/cyclin D1 has relatively good sensitivity (50%-70%) and specificity for mantel cell lymphoma.
CD38 (Fig. 13) and CD138 (Syndecan-1) (Fig. 14) are expressed in normal plasma cells and in 60% to 100% of multiple myelomas and plasmablastic lymphomas; they are found, however, in less than 5% of other large-cell lymphomas with plasmacytic differentiation.15
The same specimen as that shown in Figure 13 studied immunohistochemically with CD138. Note the expression of this marker in the neoplastic plasma cells.
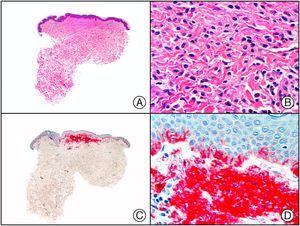
CD10, also known as neprilysin or neutral endopeptidase, is a cell-surface glycoprotein expressed in a wide variety of normal tissue, such as the brush border of enterocytes in the upper gastrointestinal tract, bile canaliculi, glomerular epithelial cells in the kidney and the brush border of proximal tubular cells, myoepithelial cells of the breast and salivary and sweat glands, placental trophoblasts, and in a minority of myofibroblasts (including cutaneous periadnexal cells). It is also detected in apocrine metaplasia of the breast. In the bone marrow, it is expressed on the surface of stem cells and in myelopoietic cells (including neutrophils). In non-neoplastic lymphoid tissue, CD10 is strongly expressed in follicle center cells (secondary follicles). It is also found in certain mature B cells and in a subpopulation of parafollicular T cells. It stains normal germinal centers and approximately 90% of cases of pre-B ALL and blast crisis in chronic myeloid leukemia. It is positive in Burkitt lymphoma and in most FCLs as well as in some cases of diffuse large B-cell lymphoma and mantle cell lymphoma. It is, however, negative in marginal zone B-cell lymphoma.16 There have been recent reports of CD10 expression in many other cutaneous tumors of very different lineage, including dermatofibroma, dermatofibrosarcoma protuberans, squamous cell carcinoma, atypical fibroxanthoma (Fig. 15), basal cell carcinoma, trichoepithelioma, and melanoma. Finally, CD10 is also expressed in other extracutaneous tumors, such as renal cell carcinoma, endometrial carcinoma, and hepatocellular carcinoma.18
Atypical fibroxanthoma. A, Low-power magnification. B, Detail of neoplastic cells showing an atypical, pleomorphous nucleus and a wide cytoplasm with a foamy appearance. C, The same specimen studied immunohistochemically with CD10. D, Detail showing CD10 expression in many of the neoplastic cells.
CD23 is a low-affinity immunoglobulin (Ig) E receptor that is thought to participate in regulation of the IgE response and B-cell activation. It is expressed in mature B cells, including those in the mantle zone, and, at low levels, in T cells, natural killer (NK) cells, Langerhans cells, and platelets. Although CD23 is positive in mantle cells, it has traditionally been considered to yield negative results in mantle cell lymphoma (with positivity rates ranging from 0%-13%, depending on the series). However, recent studies have reported CD23 positivity in almost 25% of mantel cell lymphomas using more sensitive methods, such as flow cytometric immunophenotyping; it has even been suggested that CD23 expression may be associated with better prognosis.19
The study of κ and λ light-chain restriction is very useful in demonstrating the monoclonality of B-cell and plasma cell proliferations. Conventional immunohistochemistry results are often difficult to interpret due to the frequent high background noise, but greater specificity is possible using in situ hybridization. The expression of a single κ or λ chain indicates monoclonality (Fig. 16). The expression of both chains in a lymphoid infiltrate, by contrast, reveals B-cell polyclonality, which in many cases is consistent with a reactive (non-neoplastic) process.
Marginal-zone B-cell lymphoma with differentiation towards plasma cells (this entity was originally called primary cutaneous plasmacytoma). A, Low-power magnification. The homogeneous eosinophilic appearance is due to presence of abundant amyloid deposits. B, Detail of neoplastic plasma cells. C, Strong staining with κ light chains in the cytoplasm of neoplastic cells. D, Negative expression of λ light chains in neoplastic cells.
The monoclonal antibodies anti-CD2, CD3, CD4, CD5, CD7, and CD8 are the main immunohistochemical markers used to study T-cell infiltrates. CD4 is a marker of helper T cells and is expressed in most cases of peripheral T-cell lymphoma, mycosis fungoides (Fig. 17), Sézary syndrome, CD4+ medium-sized T-cell lymphoma, adult T-cell leukemia/lymphoma linked to infection by human T-cell lymphotropic virus 1 (HTLV-1), and blastic plasmacytoid dendritic cell neoplasm. The loss of expression of any of these T-cell markers (e.g., CD2, CD5, or CD7) generally indicates malignancy and can help in the diagnosis of peripheral T-cell lymphoma, although it should be noted that loss of CD7 expression may occur in inflammatory infiltrates in diseases such as psoriasis and lichenoid dermatitis.20 In the case of mycosis fungoides, infiltrates are essentially composed of mature CD4+ T cells. The detection of a high CD4/CD8 ratio and a low (generally <25%) CD8/CD3 ratio therefore aids diagnosis.
Folliculotropic mycosis fungoides. A, Low-power magnification showing an infiltrate with a perifollicular distribution. B, Details of lymphocytes dotted along the peripheral rows of the outer root sheath of the hair follicle. C, The same specimen studied immunohistochemically with CD4. Note the strong staining of the infiltrate. D, Detail of CD4-positive lymphocytes dotted through the follicular epithelium.
CD8 is a marker of cytotoxic T cells and certain NK cells. Within the category of primary cutaneous T-cell lymphomas, CD8 is expressed in neoplastic cells in panniculitis-like T-cell lymphoma (Fig. 18), epidermotropic CD8+ T-cell lymphoma, and certain types of γ-δ T-cell lymphoma.15
Subcutaneous panniculitic T-cell lymphoma. A, Low-power magnification showing more intense involvement of the subcutaneous tissue. B, Atypical lymphocytes with a hyperchromatic nucleus around the necrotic adipocytes. C, The same specimen studied immunohistochemically with CD8. D, Detail of CD8-positive neoplastic lymphocytes. Note the presence of cytophagocytosis.
CD43 is an antibody that targets sialophorin and stains both normal and neoplastic T cells. It is expressed in almost all cases of acute myeloid leukemia and in most T-cell lymphomas, and is aberrantly expressed in several cases of marginal zone B-cell lymphoma and chronic B-cell lymphocytic leukemia (Fig. 19). It is also useful in the identification of T cells when added to panels of pan B-cell markers.15
The same specimen as that shown in Figure 8 with cutaneous infiltration by chronic B-cell lymphocytic leukemia, with aberrant CD43 expression.
CD56 is a neural cell adhesion marker that is also expressed in NK cells. The antibody recognizes extranodal NK/T-cell lymphoma, nasal type (Fig. 20); plasmacytoid dendritic cell neoplasm; and a small subset of other aggressive T-cell lymphomas.
Natural killer T-cell lymphoma, nasal type. A, Low-power magnification showing a diffuse infiltrate throughout the dermis. B, High-power magnification showing medium-sized pleomorphic lymphocytes. C, The same specimen studied immunohistochemically with CD56. D, Detail of CD56-positive neoplastic lymphocytes.
Granzyme, perforin, and TIA-1 (T cell–restricted intracellular antigen 1) are all cytotoxic T-cell markers.
The transmembrane protein CD25 is a marker of the α unit of the interleukin (IL) 2 receptor and is expressed in activated T cells and B cells, some thymocytes, myeloid precursor cells, and oligodendrocytes. It is expressed in the majority of B-cell tumors, certain types of ALL, neuroblastomas, and lymphocytic tumor infiltrates of different lineage. Its soluble form, known as sIL-2R, tends to be expressed at high levels in these tumors, and serum levels are occasionally used to monitor disease progression. CD25 is also expressed in HTLV1+ T-cell lymphoma and in a subset of mycosis fungoides.
CD30/Ki-1 is expressed in both T cells and activated B cells. It recognizes anaplastic large-cell lymphoma, lymphomatoid papulosis (Fig. 21), transformation of mycosis fungoides to large-cell lymphoma, and Hodgkin disease. However, it is also expressed in activated lymphoid cells in the infiltrate of certain reactive skin disorders, such as scabies, reactions to insect bites, and certain drug-induced rashes, as well as in the infiltrates of viral infections, such as those caused by HSV, ZHV, orf virus, and molluscum contagiosum.21–24 Therefore, CD30 staining patterns in a cutaneous lymphoid infiltrate should be interpreted with caution. Staining of clusters of cells is characteristic of neoplastic disease, while that of isolated cells dotted through the infiltrate tends to be seen in reactive disease.25–27 Clinical manifestations are essential for differentiating between anaplastic large-cell lymphoma (whether primary cutaneous tumors or secondary tumors that have spread to the skin) and lymphomatoid papulosis, but CD30 is generally expressed in over 75% of neoplastic cells in both primary and secondary anaplastic large-cell lymphoma; the percentage of CD30-positive cells tends to be lower in lymphomatoid papulosis.15
CD21 and CD35 are markers of complement receptors, C3d and C3b, and are expressed in follicular dendritic cells and associated neoplasms.
Finally, βF1 (TCRβ chain) is a specific and relatively sensitive marker of T-cell neoplasms with an α/β immunophenotype; clinically these tumors are much less aggressive than those that express γ/δ receptors.
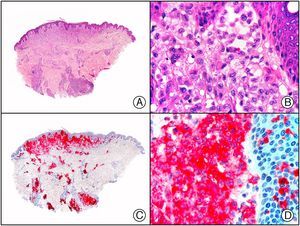
CD123 is a marker of the IL-3 receptor α-chain. This cytokine promotes cell-cycle progression and differentiation and inhibits hematopoietic cell apoptosis. It is expressed in precursor myeloid cells, macrophages, plasmacytoid dendritic cells, mastocytes, basophils, and megakaryocytes. It is also strongly expressed in numerous leukemic blasts and leukemia stem cells and would therefore appear to be an excellent therapeutic target.28 Recent studies have shown high CD123 expression in the stem cells of patients with acute myeloid leukemia, and found a correlation with poorer prognosis.29 CD123 is also highly expressed in the bone marrow of patients with myelodysplastic syndrome, and is useful in the diagnosis of B-cell disorders with circulating villous lymphocytes, as it is typically expressed in hairy cell leukemia.30 Its most widespread application, however, is perhaps in the diagnosis of blastic plasmacytoid dendritic cell neoplasm31 (Fig. 22).
Blastic plasmacytoid dendritic cell neoplasm. A, Low-power magnification showing a diffuse infiltrate throughout the dermis. B, Characteristics of neoplastic cells, showing a monomorphous infiltrate consisting of medium-sized blastic cells. C, The same specimen studied immunohistochemically with CD123. D, Detail of CD123-positive neoplastic cells.
The coexpression of myeloperoxidase, lysozyme, CD45, CD43, and CD74 supports a diagnosis of cutaneous infiltration by neoplastic cells of myeloid lineage. However, it should be noted that the negative expression of myeloid markers, such as lysozyme and myeloperoxidase, and the aberrant expression of T-cell markers, such as CD45RO, in certain cases of infiltration by leukemic cells can give rise to diagnostic errors. Furthermore, these markers are not exclusive to leukemic skin infiltrates, and are also seen in reactive disorders, such as histiocytoid Sweet syndrome32 (Fig. 23), in which the dermal infiltrate is composed of immature myeloid cells (granulocyte precursors), which have a very similar immunophenotype to that seen in true myeloid leukemia skin infiltrates.
Histiocytoid Sweet syndrome. A, Low-power magnification showing a bandlike infiltrate in the superficial dermis. B, Detail of mononuclear cells in the infiltrate. C, The same specimen studied immunohistochemically with myeloperoxidase. D, Detail of myeloperoxidase staining in many of the mononuclear cells of the infiltrate.
Anti-CD68 antibody recognizes a 110-kDa protein in the cell cytoplasm, and more specifically in lysosomes. It yields positive results in different types of cells, including myeloid, monocytic, and histiocytic cells, and their respective tumors.33 It stains monocytic macrophages, myeloid precursor cells in the bone marrow, histiocytes in normal lymphoid tissue, and Kupffer cells, although it is also expressed in mast and microglial cells.34 CD68 is positive in histiocytic tumors, such as juvenile xanthogranuloma (Fig. 24), as well as in Langerhans cell histiocytosis and certain subtypes of myeloid leukemia (depending on the antibody used). It is, however, also expressed in several epithelial tumors and in the epithelioid cells of certain melanomas. It is positive in over 70% of melanomas and other skin tumors, such as angiosarcoma, atypical fibroxanthoma, squamous cell carcinoma, and leiomyosarcoma. Of the 2 commercial clones available for this antibody, PGM-1 (phosphoglucomutase 1) and Kp-1, PGM-1 has greater specificity for the recognition of histiocytic cells.
Compared with CD68, CD163 has high specificity for the identification of cells in the monocyte-macrophage system (Fig. 25). However, it is not a good marker of myeloid sarcoma or of acute myeloid leukemia of monocytic lineage.34
CD34, as already mentioned, is a transmembrane glycoprotein expressed mainly in endothelial cells, dendritic fibroblasts, and hematopoietic stem cells. It is generally expressed in only 1.5% of bone marrow cells and in less than 0.5% of peripheral blood cells. Erythroid, myeloid, and megakaryocytic cells are CD34-positive, as are immature TdT-positive lymphoid cells. CD34 is thus a good marker of acute leukemia and precursor cells harvested for bone marrow transplantation. In the category of hematopoietic neoplasms, it is expressed in acute B-cell and T-cell lymphoblastic leukemia and acute myeloblastic leukemia.35–37 In myelodysplastic syndrome, it is a predictor of transformation, and therefore of poor prognosis. Recent studies have also confirmed its value as a marker of poor prognosis in acute myeloid leukemia.38
CD15 (Leu M1) reacts with antigens in mature neutrophils, monocytes, and a subset of T cells. It is a known marker of Reed-Sternberg cells in classic Hodgkin lymphoma, but is not expressed in nodular lymphocyte-predominant Hodgkin lymphoma. It is negative in most non-Hodgkin lymphomas, with the exception of some anaplastic large-cell lymphomas, above all those affecting the skin, and some peripheral T-cell lymphomas. It is expressed in 60% of adenocarcinomas. CD15 is of great value in the diagnosis of leukemia, as it recognizes practically all myeloid neoplasms, although staining patterns vary according to the antibody used. CD15 loss has been described in recurrences of certain acute myeloid leukemias, and has therefore been linked to poor prognosis. It is positive in almost all cases of chronic myeloid leukemia during the chronic phase and in approximately 50% of ALLs. Its levels are higher in common ALL antigen (CALLA)-negative cases, which are generally associated with poorer prognosis than CALLA-positive cases. Like CD34, the antibody stains neoplastic cells of granulocytic lineage.39
Mast CellsCD117 (c-Kit) and tryptase are the most useful immunohistochemical markers of mast cells. The proto-oncogene c-kit encodes a 145-kDa transmembrane receptor with tyrosine kinase activity that participates in hematopoiesis, gametogenesis, and melanogenesis. It is closely related to malignant transformation and the pathogenesis of several tumors. CD117 and tryptase are positive in the majority (>95%) of neoplastic cells in all types of mast cell disease40 (Fig. 26).
Urticaria pigmentosa. A, Low-power magnification. B, Cytologic features of mast cells infiltrating the dermis. C, The same specimen studied with CD117. D, Detail of high CD117 expression in the dermal mast cells. Note also the staining of the dendritic melanocytes at the dermal-epidermal junction.
CD1a, a surface antigen expressed by Langerhans cells, is useful in the diagnosis of Langerhans cell histiocytosis. It should be noted, however, that Langerhans cells can be present in very large numbers in reactive disorders.15
CD207 (langerin) is a highly specific marker of Langerhans cells (even more so than CD1a), as it stains Birbeck granules and is positive in Langerhans cell histiocytosis (Fig. 27) and in a subset of histiocytic sarcomas.41
Langerhans cell histiocytosis. A, Low-power magnification. B, Detail of cytologic characteristics of Langerhans cells in the infiltrate. C, The same specimen studied immunohistochemically with CD207 (langerin). D, Detail of CD207-positive cells in the infiltrate. Note also the expression of CD207 in isolated intraepidermal cells.
p53 antibody recognizes the N-terminal epitope of protein p53, which is encoded by the suppressor gene p53 on chromosome 17. Its immunohistochemical detection is associated with mutations and it is used as a predictive marker in different types of cancer.
The retinoblastoma RB1 gene encodes the synthesis of the nuclear phosphoprotein, pRb, which plays a critical role in cell cycle control by interacting with the transcription factor E2F. Loss of pRb expression has been reported to have prognostic value in certain neoplasms.
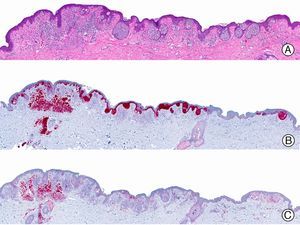
p16 (INK4a) is a protein encoded by the suppressor gene CDKN2, which participates in the regulation of the G1 phase of the cell cycle. CDKN2 mutations increase the risk of developing a variety of tumors, in particular melanoma, as they cause loss of tumor-suppressor function. The expression of p16 is strictly regulated in normal cells, in which the levels are very low and not detected by immunohistochemistry. The protein is, however, expressed in benign melanocytic lesions, such as Spitz nevus. Loss of expression (Fig. 28), together with high levels of a proliferation marker (e.g., Ki-67) and p53 overexpression is associated with progression in melanoma.
Melanoma with large melanocytic nests at the dermal-epidermal junction. A, Low-power magnification. B, The same specimen studied immunohistochemically with S-100 protein. C, The same specimen studied immunohistochemically with p16. Note the loss of p16 expression in many of the nests at the dermal-epidermal junction.
As already mentioned, S-100 protein is one of the most sensitive markers of melanocytic tumors. Other markers, however, such as Melan-A, HMB-45 (human melanoma black-45), MiTF-1 (microphthalmia transcription factor-1), SOX-10 (Sry-related HMG-BOX gene-10), and tyrosine, are necessary, as S-100 protein is not exclusive to these tumors. The study of proliferation markers, such as Ki-67 and pHH3 (phosphohistone 3), is also very useful for distinguishing between benign and malignant tumors. Several of these markers have already been discussed, and Table 2 summarizes the main applications of the different immunohistochemical markers used in the study of melanocytic tumors.
Immunohistochemical Markers Used in Melanocytic Tumors.
| Marker | Pattern | Application |
| S-100 | Nuclear/cytoplasmic | Greatest sensitivity for melanoma and spindle cell melanoma/desmoplastic melanoma |
| HMB-45 | Cytoplasmic | More specific than S-100 for melanoma; useful for distinguishing between melanoma and melanocytic nevus |
| Melan-A | Cytoplasmic | Similar sensitivity and specificity to HMB-45, more diffuse, intense staining |
| Tyrosinase | Cytoplasmic | High sensitivity and specificity for melanoma, but reduced sensitivity at more advanced stages and in metastases |
| Ki-67 | Nuclear | Stains <5% of cells in melanocytic nevi and 13%-30% of cells in melanoma; it also stains a considerable percentage of cells in Spitz nevus |
| SOX-10 | Nuclear | More sensitive than S-100, as it stains S-100-negative melanoma; useful for distinguishing between desmoplastic melanoma and proliferation of melanocytes in surgical scars |
| MiTF-1 | Nuclear | High sensitivity but lower specificity; very useful for identifying the nuclei of melanocytes in highly pigmented melanocytic tumors after bleaching |
Source: Adapted from Prieto et al.45
Abbreviations: HMB-45, human melanoma black 45; MiTF-1, microphthalmia transcription factor 1; S-100, S-100 protein; SOX-10, Sry-related HMG-BOX gene 10.
Tyrosine is an enzyme involved in the final step of melanin biosynthesis. Thanks to its specificity and high sensitivity, it is an adequate marker of cells of melanocytic origin, together with S-100 protein, HMB-45, and Melan-A. Its diagnostic sensitivity in melanoma, however, is lower in more advanced tumors and in metastases.1
CD99 was recently proposed as a useful marker for distinguishing between Spitz nevus and melanoma,1 but further studies are required. Perhaps more interesting in this respect are the findings recently reported by Garrido-Ruiz et al.,42 who studied a panel of markers of cell cycle, apoptosis, DNA-repair proteins, and membrane receptors in 28 Spitz nevi and 62 primary vertical growth phase melanomas. The results showed overexpression of cyclin D2 and p21 in Spitz nevus compared with melanoma, higher expression of the proliferation markers Ki-67 and topoisomerase IIα in deep areas of melanomas compared with Spitz nevi, and higher expression of nuclear survivin in melanomas. These 5 markers would, therefore, appear to be more useful than C99 for distinguishing between melanoma and Spitz nevus.
COX-2 (cyclooxygenase 2) is an inducible enzyme involved in the production of prostaglandins in several inflammatory processes. It is believed to play an important role in the pathogenesis of tumors in different organs, including the colon, the rectum, the stomach, the breast, the bladder, and the lungs. It is also overexpressed in malignant skin tumors, such as squamous cell carcinoma, basal cell carcinoma, Bowen disease, actinic keratosis, and malignant melanoma. Recent studies have shown much higher COX-2 expression in melanoma compared with melanocytic nevi, indicating that COX-2, used in combination with other markers, would be of great help in the complex task of distinguishing between benign and malignant melanocytic tumors.43
As already discussed in the section on B-cell markers, MUM-1 is a 50-kDa nuclear marker, encoded by MUM1, that was originally identified in multiple myeloma.1 It has since, however, also been detected in melanoma and its metastases, but not in other malignant tumors. It is also strongly expressed in benign melanocytic nevi, including Spitz nevus, but not in desmoplastic melanoma. It therefore has high sensitivity for certain melanocytic tumors.44
Ki-67 (Mib-1) is the most widely used proliferation marker. It can be used in practically all tumors and is very useful for discerning between benign and malignant melanocytic lesions. It is generally expressed in the nucleus of less than 5% of neoplastic cells in benign melanocytic lesions, but higher levels tend to be seen in cells at the dermal-epidermal junction. Special caution should be taken not to interpret staining of the nuclei of basal epidermal keratinocytes as positive, as these cells generally have high proliferative activity.45 Over 50% of neoplastic cells in malignant melanocytic tumors are generally Ki-67-positive, and it is not uncommon to observe nuclear staining in many cells in the deep areas of the lesion (Fig. 29).
Nodular epithelioid cell melanoma. A, Low-power magnification. B, Detail of cytologic characteristics of neoplastic cells showing pleomorphic nuclei and an ample eosinophilic cytoplasm. C, The same specimen studied immunohistochemically with Ki-67. D, Detail showing Ki-67 staining in the nuclei of many neoplastic cells.
pHH3 is probably the most specific marker of mitosis. Histone H3 is a nuclear protein, whose phosphorylation reaches maximum levels during mitosis and minimum levels during interphase. Phosphorylation does not occur during apoptosis. pHH3 is therefore a highly sensitive marker of mitosis (Fig. 30), although it is not specific to any one cell type. To avoid confusion, it is sometimes necessary to use a second, cell-specific, marker. The findings of recent studies lend support to the value of pHH3 in the study of melanocytic tumors.1
Elastin was recently proposed as a marker for differentiating between melanocytic nevus and melanoma, as it is expressed in the dermal nests of the former but not of the latter.46
It is sometimes necessary to distinguish histologically between pigmented lesions in melanoma surgical scars, the presence of residual melanoma after excision, and desmoplastic melanoma. S-100 protein is expressed in the melanocytes of all lesions. However, the detection of tumor cells expressing HMB-45, MiTF, or Melan-A should point to a diagnosis of residual melanoma. The problem is that these 3 markers are generally negative in desmoplastic melanoma. The value of S-100 protein and SOX-10 in desmoplastic melanoma has already been discussed. p75/NGFR (nerve growth factor receptor) is also expressed in most desmoplastic and neurotropic melanomas (Fig. 31), but at higher levels than S-100 protein.15,45
Desmoplastic melanoma. A, Low-power magnification showing a diffuse infiltrate throughout the dermis dotted with lymphoid nodules. B, Detail of spindle-cell morphology of neoplastic cells. C, The same specimen studied immunohistochemically with p75. D, Detail of p75-positive neoplastic cells.
Approximately 50% of melanoma metastases that are negative for S-100 protein are positive for MiTF1 and/or SOX-10, meaning that the combined use of these markers may be useful in certain cases. The use of KBA-62 was also recommended in a recent study, as it appears to be expressed in the majority of desmoplastic and spindle cell melanomas and in up to 91% of melanoma metastases.47
As mentioned in the first part of this review, it is sometimes very difficult to distinguish between pigmented actinic keratosis and malignant melanoma in situ in skin with actinic damage. Melan-A stains both dendritic melanocytes and some pigmented basal keratinocytes, and results should therefore be interpreted with caution in such cases. In melanoma, one generally observes the staining of melanocytes arranged in confluent nests or forming a continuous border along the dermal-epidermal junction. In skin with chronic actinic damage or in pigmented actinic keratosis, by contrast, one generally observes isolated atypical melanocytes dotted along the basal layer and in the upper layers of the epidermis. Nevertheless, it is better not to use Melan-A in this setting. S-100 protein, HMB-45, and SOX-10 are more useful and easier to interpret, as they stain dendritic melanocytes in true melanoma in situ but not atypical pigmented keratinocytes in pigmented actinic keratosis.45,48
Spindle Cell TumorsIn undifferentiated skin tumors, which are often located on the face of elderly patients with considerable chronic actinic damage, it is very difficult to distinguish histologically between different forms of cutaneous spindle cell tumors using conventional histologic techniques. In most cases, a definitive diagnosis can be established only by immunohistochemistry (Table 3).
Immunohistochemical Differential Diagnosis for Cutaneous Spindle Cell Tumors.
| Marker | Melanoma | Atypical Fibroxanthoma | Sarcomatoid Squamous Cell Carcinoma | Leiomyosarcoma |
| S-100 | + | − | − | − |
| CK903 | − | − | + | − |
| p63 | − | ± | + | ± |
| CD10 | ± | + | ± | Unknown |
| Desmin | − | − | − | + |
Source: Adapted from Hoang et al.15
Abbreviations: CK, cytokine; S-100, S-100 protein.
Spindle cell (sarcomatoid) squamous cell carcinoma is a poorly differentiated squamous cell carcinoma that usually expresses CK903, CK34βE12, CK5/6, and MNF-116. However, it also expresses vimentin, which when detected in conjunction with spindle cell morphology, can lead to a misdiagnosis of superficial sarcoma. p63 has proven to be a useful marker for discerning between spindle cell squamous cell carcinoma, fibroxanthoma, and cutaneous leiomyosarcoma, as it is positive in the majority of spindle cell squamous cell carcinomas, but in just 20% of fibroxanthomas and 50% of leiomyosarcomas.15
Fibroxanthoma is usually diagnosed by exclusion, following the ruling out of poorly differentiated squamous cell carcinoma (negative CK expression), spindle cell melanoma (negative S-100 protein and SOX-10 expression), and cutaneous leiomyosarcoma (negative actin and desmin expression). Most fibroxanthomas express histiocytic markers, such as CD68. CD99 has also proven useful, as there are reports of CD99 positivity in up to 73% of fibroxanthomas but in only 10% of desmoplastic melanomas; it is not found in spindle cell squamous cell carcinoma.49 CD10 yields strong diffuse staining in most neoplastic cells in fibroxanthoma (Fig. 15). However, results should be interpreted with caution when studying cutaneous spindle cell tumors, as CD10 has also been observed in melanoma, dermatofibrosarcoma protuberans, and spindle cell carcinoma.18 Smooth muscle actin is positive in 45% of fibroxanthomas, while desmin is almost always negative.15
CD34 and factor XIIIa are the most useful markers for distinguishing between dermatofibroma and dermatofibrosarcoma protuberans. Factor XIIIa is expressed in the majority of dermatofibromas, but is negative in dermatofibrosarcoma protuberans. CD34, by contrast, is expressed in the majority of proliferating cells in dermatofibrosarcoma protuberans and negative in those in dermatofibroma. However, it should not be forgotten that the discriminatory power of these 2 antibodies is not absolute, as focal CD34 staining has been observed in the periphery of some dermatofibromas, particularly in deep, densely cellular lesions. There have also been reports of dermatofibrosarcoma protuberans not expressing CD34.15
Superficial acral fibromyxoma is composed of spindle cells that may be focally arranged in a storiform pattern, suggesting a possible diagnosis of dermatofibrosarcoma protuberans. CD34 is expressed in the neoplastic cells of both tumors, but ApoD (apolipoprotein D) is found only in dermatofibrosarcoma protuberans. EMA (epithelial membrane antigen) and CD99 tend to be positive in superficial acral fibromyxoma and negative in dermatofibrosarcoma protuberans.
Cellular digital fibroma is another benign tumor formed by monomorphous spindle cells arranged in a storiform pattern, immersed in a stroma with abundant collagen; it is positive for CD34. The histologic findings may therefore suggest a diagnosis of dermatofibrosarcoma protuberans. However, with an adequate biopsy specimen that includes tumor margins, it is easy to distinguish between the 2 entities, as cellular digital fibroma is a superficial, well-delimited lesion that is confined to the reticular dermis. Dermatofibrosarcoma protuberans, by contrast, can extend to the subcutaneous tissue. Immunohistochemically, spindle cells in cellular digital fibroma, apart from being strongly positive for CD34, are also positive, to varying degrees, for factor X111a and generally negative for EMA and CD99.50
Sebaceous TumorsAs already mentioned, EMA stains the sebaceous gland in normal skin; it labels both the excretory duct and sebocytes (with staining of cytoplasmic microvacuoles) in sebaceous lobules. The same occurs in tumors with sebaceous differentiation. It should be noted, however, that EMA is also positive in squamous cell carcinoma and other adnexal tumors, meaning that other markers may on occasions be necessary to confirm sebaceous differentiation.
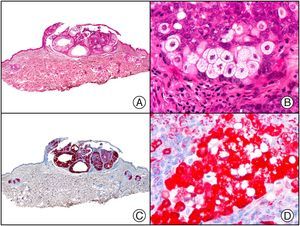
Adipophilin is a monoclonal antibody that is very useful in the identification of intracytoplasmic lipids, such as those found in sebocytes. It is particularly useful in the identification of poorly differentiated sebaceous carcinomas, and in difficult cases of periocular sebaceous carcinoma with small biopsy specimens. It is essential to carefully observe the staining pattern produced by adipophilin. It specifically stains neoplastic sebaceous cells in the membrane delimiting multiple, small intracytoplasmic lipid vacuoles (Fig. 32), but weak focal staining and granular morphology may also be observed in other normal cells and in certain nonsebaceous clear-cell tumors. Adipophilin is also expressed in foamy histiocytes in xanthomatous lesions and in epithelial cells of cutaneous metastases from renal carcinoma, which are characterized by intracytoplasmic glycogen and multiple lipid vacuoles. Adipocytes and their respective tumors, however, are adipophilin-negative, as this antibody does not stain the membrane of the only large lipid vacuole occupying the entire cytoplasm of adipocytes.51
Sebaceous adenoma, sebaceous epithelioma, and sebaceous carcinoma are all uncommon tumors, but it is important to diagnose them correctly as they are cutaneous manifestations of Muir-Torre syndrome. Muir-Torre syndrome is caused by a germline mutation in 1 or more of the DNA mismatch repair (MMR) genes MSH2, MLH1, MSH6, and PMS2. Screening for these genes can be performed immunohistochemically in formalin-fixed or even paraffin-embedded sections. It has been estimated that the combined study of MMR proteins has a positive predictive value of 55% for the diagnosis of Muir-Torre syndrome in sebaceous tumors that exhibit loss of MSH2 (Fig. 33) and MSH6, and of 100% in tumors that exhibit loss of MLH1 and MSH6, or of all 3 markers.52–54
Cystic sebaceous carcinoma in a patient with Muir-Torre syndrome. A, Low-power magnification. B, Detail of a group of sebocytes at different stages of maturation. C, The same specimen studied immunohistochemically with MSH2. Note how the nuclei of the epidermal keratinocytes express MSH2 as a positive control. D, High-power magnification showing how the nuclei of neoplastic cells in this sebaceous carcinoma do not express MSH2.
Most reports of cutaneous eccrine and apocrine adnexal tumors in the literature use the term eccrine to describe all types of adnexal tumors. Eccrine poroma, eccrine spiradenoma, and eccrine syringoid carcinoma are good examples of this. Nonetheless, to date, nobody has established clear and precise histopathologic criteria to describe what constitutes an eccrine tumor. In theory, a tumor is said to display eccrine differentiation when it reproduces, with more or less success depending on its degree of differentiation, a structure of the normal eccrine gland. However, perhaps with the exception of eccrine mixed tumor, there have been no reports of tumors reproducing the secretory portion of eccrine glands, i.e. of tumors comprised of glandular structures lined with a single row of columnar epithelial cells with alternating clear and dark cells and a discontinuous peripheral row of myoepithelial cells. However, the greatest problem is posed by ductal tumors, which constitute, by far, the largest proportion of tumors with eccrine or apocrine differentiation. It is currently impossible to know whether a particular ductal tumor is eccrine or apocrine, simply because the eccrine and apocrine ducts are, to date, histologically, immunohistochemically, and ultrastructurally indistinguishable. It is only in the case of normal eccrine and apocrine glands that it can be stated for certain whether a particular duct is eccrine (when it opens directly into the epidermis) or apocrine (when it opens into the infundibulum of a hair follicle). However, in the majority of ductal tumors, regardless of whether they are benign or malignant, all that can be said for certain is that they are ductal tumors. Any additional designation, such as eccrine or apocrine, is pure conjecture. Immunohistochemistry emerged as a possible solution to this problem and several markers have been proposed for distinguishing between eccrine and ductal differentiation. However, in practice, none of these markers have provided a reliable, reproducible way of differentiating between eccrine and apocrine ducts in normal eccrine and apocrine glands, meaning that their usefulness in differentiating between apocrine and eccrine ductal tumors is still questionable. Table 4 shows the main immunohistochemical markers used to study differentiation in cutaneous adnexal tumors.
Most Common Immunohistochemical Markers Used to Investigate Adnexal Tumor Differentiation.
| Marker | Pattern | Application |
| CEA | Cytoplasmic | Marker of glandular differentiation; can help distinguish extramammary Paget disease |
| GCDFP-15 | Cytoplasmic | Marker of apocrine differentiation, although also stains eccrine glands |
| EMA | Cytoplasmic | Stains malignant eccrine tumors and occasionally apocrine tumors, as well as most sebaceous glands |
| CK7 | Cytoplasmic | Sensitive and specific for Paget and extramammary Paget disease |
| AE1/AE3 | Cytoplasmic | Pancytokeratin marker used in glandular tumors |
| Calretinin | Cytoplasmic | Marker of innermost layer of outer root sheath, sebaceous duct, and eccrine secretory portion; it is expressed in proliferations with trichilemmal, sebaceous ductal, and secretory eccrine differentiation |
| CD34 | Cytoplasmic | Differentiation towards outer root sheath of the hair follicle (trichilemmal differentiation); also stains periadnexal dermal fibrocytes |
| Adipophilin | Cytoplasmic, vacuolar | Marker of sebaceous differentiation |
| BerEP4 | Cytoplasmic | Positive in basal cell epithelioma and negative in basal cell carcinoma and trichoblastoma; also stains sebaceous tumors |
| Bcl-2 | Cytoplasmic | Stains the basal layer of the epidermis; can be helpful in distinguishing basal cell epithelioma from trichoepithelioma |
Source: Adapted from Wasserman et al.1
Abbreviations: Bcl, B-cell lymphoma; CEA, carcinoembryonic antigen; CK, cytokeratin; EMA, epithelial membrane antigen; GCDFP-15, gross cystic disease fluid protein 15.
A number of differential diagnoses deserve special mention. At times, the detection of cords of basaloid epithelial cells immersed in a desmoplastic stroma can suggest a diagnosis of morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, undifferentiated trabecular squamous cell carcinoma, microcystic adnexal carcinoma, or syringoid carcinoma. Most basal cell carcinomas are positive for CD10, but morpheaform basal cell carcinoma exhibits only weak staining for CD10 in tumor stromal cells. Stromal fibrocytes in desmoplastic trichoepithelioma also express CD10.55 In such cases, immunostaining with BerEp4 can be useful, as this marker stains the neoplastic epithelium of almost all histopathologic variants of basal cell carcinoma (Fig. 34), but not that of trichoblastoma or squamous cell carcinoma.56–59 CEA (carcinoembryonic antigen) and EMA can be used to identify small ductal formations in microcystic adnexal carcinoma and syringoid carcinoma.
The histopathologic distinction between primary adnexal carcinoma and cutaneous metastasis from a visceral adenocarcinoma is also a common problem. CK5/6, p63, and podoplanin have been proposed as markers for such cases, as they are preferentially expressed in primary carcinomas but tend to be negative in metastases arising from visceral adenocarcinomas.60 Nonetheless, the discriminatory capacity of these markers is not absolute, as recent studies have shown p63 to be expressed with relative frequency in cutaneous metastases from adenocarcinoma, which also exhibit focal podoplanin positivity in up to 5% of cases.61,62 GCDFP-15 (gross cystic disease fluid protein 15) has a sensitivity and specificity of 99% for metastases from breast adenocarcinoma, but is also positive in cutaneous eccrine and apocrine carcinomas.63
Immunohistochemical Study of Skin MetastasesTable 5 shows the immunohistochemical features of most cutaneous metastases from malignant tumors.64
Immunohistochemical Markers Used in Investigating the Origin of Cutaneous Metastases from Internal Malignant Tumors.
| Origin | Histopathology | Immunohistochemical Markers | |
| Positive | Negative | ||
| Breast | Ductal carcinoma | CK7, estrogen receptors, progesterone receptors, GCDFP-15, CEA, c-erb-B2, mammaglobin, E-cadherin | CK20, CK5/6 |
| Lobular carcinoma | CK7, estrogen receptors, progesterone receptors, GCDFP-15, CEA, EMA, mammaglobin | S-100, E-cadherin, podoplanin, p63 | |
| Inflammatory carcinoma | CD31, podoplanin | ||
| Telangiectatic carcinoma | CD31 | Podoplanin | |
| Paget disease | MUC-1, CK7 | MUC2, MUC5AC, CK20 | |
| Lung | Squamous cell carcinoma | CK5/6 | CK7, CK20, TTF-1, CEA |
| Adenocarcinoma | CK7, TTF-1, Ber-EP4, CEA, surfactant apoprotein A | CK5/6, CK20 | |
| Small-cell carcinoma | TTF-1, CAM 5.2, CK8/18, BerEP4, NSE | CK7, CK20, CD99 | |
| Mesothelioma | CK5/6, calretinin, vimentin | CEA, TTF-1, S-100, CD31 | |
| Colorectal | Adenocarcinoma | CK20, CEA, CDX2 | CK7 |
| Small intestine | Squamous cell carcinoma | CK20, EMA, AE1/AE3 | CK7 |
| Adenocarcinoma | CEA, EMA | ||
| Stomach | Adenocarcinoma | CK20, CEA, EMA, CDX2, HIK1083 | CK7+/- |
| Esophagus | Squamous cell carcinoma/adenocarcinoma | CK5/6, BerEP4 | CK7, CK20 |
| Liver | Hepatocellular carcinoma | α-fetoprotein, polyclonal CEA, Her Par-1, arginase-1 | CKs, EMA |
| Monoclonal, CEA | |||
| Biliary system | Adenocarcinoma | CK7, CK20 | CDX2 |
| Pancreas | Adenocarcinoma | CA19.9, CK7, CK8, CK18, CK19 | CK20 |
| Kidney | Renal carcinoma | AE1/AE3, MNF-116, CD31, RCC-Ma (>2/3), vimentin CD10, EMA, S-100, adipophilin, PAX-8 | Inhibin, Melan-A, TTF-1, CK7, CK20 |
| Bladder and urethra | Transitional epithelial carcinoma | CK7, CK19, CK20, CK14 (50%), thrombomodulin, uroplakin iii, CD10 | |
| Prostate | Adenocarcinoma | PSA, acid phosphatase, AMACR (P504S), ERG | CK7, CK20, thrombomodulin |
| Testicle | Choriocarcinoma | β-HCG | |
| Ovary | Carcinoma | CA-125, CK7, PAX-8 | CK20 (except for some mucinous carcinomas) |
| Cervix and vagina | Carcinoma | CK7, EMA+/- | CK20, CEA |
| Endometrium | Carcinoma | CK7, PAX-8 | CK20, p63, podoplanin |
| Thyroids | Papillary | Thyroglobulin, TTF-1, PAX-8 (50%) | |
| Follicular | Thyroglobulin, TTF-1, PAX-8 (50%) | ||
| Anaplastic | Thyroglobulin | ||
| Medullary | Calcitonin | Thyroglobulin, PAX-8 | |
| Carcinoid tumor | Enterochromaffin carcinoma | NSE, chromogranin, synaptophysin, CDX2 (intestinal origin), TTF-1 (pulmonary origin) | CK5/6, CK7, CK20, p63 |
| Neuroblastoma | NSE, chromogranin, synaptophysin, peripherin, α-internexin, MAB-1B | CD99, desmin, myogenin | |
| Soft-tissue sarcomas | Leiomyosarcoma | SMA, desmin | |
| Rhabdomyosarcoma | Desmin, SMA, Myo-D1, vimentin, myogenin, CLA (CD45) | ||
| Angiosarcoma | CD31, ERG, podoplanin, c-Myc amplification | ||
| In epithelioid variants: CKs and EMA | |||
| Chondrosarcoma | CD99, vimentin, S-100 (focal), podoplanin, YKL-40, SMA, desmin, CAM 5.2 | AE1/AE3, SMA, CD117 | |
| Ewing sarcoma | Vimentin, NSE, CD99, Leu7/CD57, FLI-1 | Podoplanin | |
| Osteosarcoma | Ezrin, SMA, C-erbB-2 (50%) | ||
| Salivary glands | Cystic adenoid carcinoma | CKs, CEA, S-100, CD117/c-Kit, SMA, vimentin (in myoepithelial component) | |
| Mucoepidermoid carcinoma | CK5/6, EMA, CEA | ||
| Larynx and trachea | Carcinoma | AE1/AE3, p53 | |
| Nasopharynx | Carcinoma | EBV RNA | |
| Glioblastoma | GFAP, EGFR (±), vimentin, YKL-40 | Neurofilaments, HMB-45, Melan-A, CKs | |
| Medulloblastoma | Synaptophysin, NSE | Neurofilaments | |
| Meningioma | Vimentin, EMA, S-100, progesterone receptors, CKs, CEA | ||
| Adrenal gland | Carcinoma | NSE, inhibin, synaptophysin (focal), A103, chromogranin, calretinin | Melan-A, CD10 |
Source: Adapted from Alcaraz et al.64
Abbreviations: MACAR, alpha-methylacyl-CoA racemase; CEA, carcinoembryonic antigen; CK, cytokeratin; CLA, common leukocyte antigen; EBV, Epstein-Barr virus; EGFR, endothelial growth factor receptor; EMA, epithelial membrane antigen; FLI-1, friend leukemia virus integration 1; GCDFP-15, gross cystic disease fluid protein 15; GFAP, glial fibrillary acidic protein; HCG, human chorionic gonadotropin; HMB-45, human melanoma black 45; MAB, microtubule-associated protein; NSE, neuron-specific enolase; PAX-8, paired box 8; PSA, prostate-specific antigen; RCC-Ma, renal cell carcinoma marker; S-100, S-100 protein; SMA, smooth muscle actin; TTF-1, thyroid transcription factor 1.
In this section, we will briefly discuss the most useful markers used in this field in dermatopathology. TTF-1 (thyroid transcription factor 1) is expressed in approximately two-thirds of lung cancers and their metastases. The combined use of estrogen receptors and GCDFP-15 is very useful in the diagnosis of breast cancer. CDX-2 is an intranuclear marker expressed in the majority of colorectal carcinomas, but it is also found in a small number of other adenocarcinomas, such as gastric carcinoma, pancreaticobiliary carcinoma, and mucinous carcinoma of the ovary. Prostate-specific antigen (PSA) is used to confirm the prostatic origin of cutaneous metastases, although it is also positive in other tumors, such as metastatic melanoma. CK7 and CK20 are used as diagnostic markers in primary extramammary Paget disease and secondary extramammary Paget disease (epidermotropic spread to the anal or genital skin). PAX-8 is a transcription factor involved in the embryonic development of Müllerian structures; it is strongly expressed in cutaneous metastases from nonmucinous carcinoma of the ovary, endometrial carcinoma, and renal carcinoma. However, it is negative in metastases from cancer of the breast, lung, biliary tract, colon, and prostate, as well as in primary cutaneous adnexal adenocarcinomas and their skin metastases.65 Arginase 1 is a characteristic liver tissue enzyme that catalyzes the hydrolysis of arginine to ornithine and urea. It is expressed in normal liver tissue and in benign and malignant hepatocytic tumors, but is negative in many other tumors, including renal cell carcinoma, neuroendocrine tumors, melanoma, gastric carcinoma, and adrenocortical carcinomas.66HER-2 (neu), also known as ERBB2, is an oncogene located on chromosome 17 that is expressed in approximately 25% to 30% of breast cancers, as well as in other adenocarcinomas and transitional cell carcinomas. Its expression in breast cancer is associated with disease progression and unfavorable outcome. Patients with HER-2 amplification in breast cancer are very likely to be resistant to treatment with tamoxifen (hormone therapy). They do, however, respond better to chemotherapy combined with trastuzumab, a humanized monoclonal antibody that targets the extracellular domain of the HER-2 receptor. In dermatopathology, this marker is useful in the differential diagnosis of skin metastases and in distinguishing Paget disease from pagetoid melanoma in situ.67
Claudins are integral membrane proteins involved in the formation of tight cell junctions; they are a variable component, with specific claudins associated with specific tissues. Claudin-1 is positive in epithelial and perineural cells and negative in many of the tumors included in the differential diagnosis of perineurioma (e.g., dermatofibrosarcoma protuberans, low-grade fibromyxoid sarcoma, and fibromatosis).68 Claudin-3 is positive in epithelial cells in the lung and liver and claudin-5 is positive in endothelial cells. Claudin 18, in turn, is expressed in neoplastic cells of pancreatic adenocarcinoma but not in normal pancreatic epithelium.69
E-cadherin is a 120-kDa transmembrane glycoprotein involved in epithelial cell adhesion. Its loss of expression is associated with disease progression in several carcinomas.70,71 In melanoma, it is more strongly expressed in primary lesions than in metastases.72 In breast cancer, detection of complete E-cadherin loss, which occurs in both in situ and invasive lobular carcinoma, allows lobular carcinoma to be distinguished from ductal carcinoma, in which E-cadherin expression is maintained. The marker has also been used to differentiate between epithelioid mesothelioma and pulmonary adenocarcinoma.73
Nervous System and Neuroendocrine TumorsSeveral cutaneous tumors with neural or neuroendocrine differentiation deserve a brief mention. Table 6 summarizes the main immunohistochemical markers used in the histopathologic study of these tumors.
Immunohistochemical Markers in Neural and Neuroendocrine Neoplasms.
| Immunohistochemical Marker | Pattern | Application |
| S-100 | Nuclear/cytoplasmic | Glioma, primitive neuroectodermal tumors, schwannoma, neurofibroma, and neuronal and chondroid tumors |
| NSE | Cytoplasmic | Neuroendocrine cells, Merkel cell carcinoma |
| EMA | Cytoplasmic | Perineural cells |
| PGP 9.5 | Cytoplasmic | Pan-neuronal |
| GFAP | Cytoplasmic | Schwann cells |
| CK20 | Perinuclear | Merkel cell carcinoma |
| Neurofilaments | Perinuclear | Axons, neuroendocrine cells, Merkel cell tumor cells |
| Chromogranin | Cytoplasmic | Neuroendocrine cells, Merkel cell carcinoma |
| Synaptophysin | Cytoplasmic | Neuroendocrine cells, Merkel cell carcinoma |
| TTF-1 | Nuclear | Negative in Merkel cell carcinoma |
Source: Adapted from Wasserman et al.15
Abbreviations: CK, cytokeratin; EMA, epithelial membrane antigen; GFAP, glial fibrillary acidic protein; NSE, neuron-specific enolase; PGP 9.5, protein gene product 9.5; S-100, S-100 protein; TTF-1, thyroid transcription factor 1.
Neurofibromas contain axons that stain with neurofilament markers, while Schwann cells express S-100 protein, CD57, and myelin basic protein; fibroblasts, by contrast, express factor XIIIa. Neurofibromas also express several growth factors and their receptors, such as the neural cell adhesion molecule CD56 and endothelial growth factors. In schwannoma, neoplastic cells express vimentin, S-100 protein, and myelin basic protein, but are negative for neurofilaments and factor XIIIa. SOX-10 was recently reported to exhibit strong positivity in Schwann cells and schwannomas, but unfortunately, it is not 100% specific, as it also stains melanocytes and benign and malignant melanocytic tumors. A small number of schwannoma cells express GFAP (glial fibrillary acidic protein). The peripheral capsule surrounding the lesion in schwannoma is comprised of perineural cells that express EMA. However, it was recently demonstrated that over 50% of conventional schwannomas contain neurofilament-positive axons, in a proportion that varies from one case to the next. It is therefore currently recommended that neurofilaments should not be used in isolation to distinguish between neurofibroma and schwannoma.74 Schwannomas are strongly positive for calretinin and podoplanin, while neurofibromas are only weakly and focally positive for these markers. Nevertheless, in recent years, there have been several reports of tumors with hybrid features of neurofibroma and schwannoma75,76 and neurofibroma and perineuroma77; the line separating different tumors affecting peripheral nerve roots, therefore, does not seem to be as clear as was originally thought.
Neoplastic cells in granular cell tumors express S-100 protein, neuron-specific enolase (NSE), PGP-9.5 (protein gene product 9.5), NK1/C3, CD68, NGFR-5, calretinin (Fig. 35), GLUT-1 (glucose transporter 1),15 MiTF-1, and α-inhibin.
Neoplastic cells in soft tissue perineurioma also express vimentin, EMA (Fig. 36), claudin-1,68 and CD10.55 In desmoplastic variants, however, the majority of cells express EMA, GLUT-1, claudin-1, type-IV collagen, CD99, smooth muscle actin, muscle-specific actin, and CKs in a lower proportion.62 Neoplastic cells are typically negative for S-100 protein, CD57, GFAP, neurofilaments, desmin, NSE, and chromogranin.
There are 2 histopathologic variants of nerve sheath myxoma (neurothekeoma): the classic, or myxoid variant, and the variant known as cellular neurothekeoma. The 2 tumors have a different immunohistochemical profile. In myxoid neurothekeoma, all the immunohistochemical findings support neural, and in particular, schwannian, differentiation, as the majority of tumor cells express S-100 protein, CD57, NSE, and GFAP, and are each surrounded by type-IV collagen. CD34 and factor XIIIa are expressed in several fibroblasts in the tumor lobules, while EMA tends to be observed in some cells (perineural cells) at the periphery of the lobules. Myxoid neurothekeoma cells do not express CKs, HMB-45, α-smooth muscle actin, desmin, CEA, CD68, synaptophysin, or chromogranin. Cellular neurothekeoma, by contrast, is negative for S-100 protein, GFAP, CD57, and EMA, which are largely considered markers of neural differentiation. The histogenesis of this variant, therefore, remains a mystery. The only markers that are typically positive in cellular neurothekeoma are NK1C3 (originally considered to be exclusive to melanocytes but now known to be expressed in all cells with abundant lysosomes), PGP-9.5 (a pan-neuronal marker), and protein S100-A6. Expression of PGP-9.5 and S100-A6 protein is the only immunohistochemical finding that supports neural differentiation in cellular neurothekeoma. Several cells in cellular neurothekeoma also express NSE and MiTF-1, but results vary from one case to the next. In summary, while immunohistochemical findings support the idea that myxoid neurothekeoma is mostly composed of Schwann cells, the true nature of neoplastic cells in cellular neurothekeoma remains unknown.78–85Finally, the immunohistochemical profile of Merkel cell carcinoma has already been discussed. In brief, primary cutaneous lesions are negative for TTF-1, which allows primary Merkel cell carcinoma to be distinguished from cutaneous metastasis from small-cell lung cancer, which can exhibit very similar histologic findings. It has also been recently postulated that MASH1, a key gene in the embryonic development of brain cells and the neuroendocrine system, may be useful for distinguishing cutaneous metastases from small-cell lung cancer from Merkel cell carcinoma, particularly in the few cases when the latter is positive for TTF-1. MASH1 is negative in primary cutaneous Merkel cell carcinoma but positive in the majority of small-cell lung cancers.86
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of DataThe authors declare that no private patient data are disclosed in this article.
Right to Privacy and Informed ConsentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fuertes L, et al. Inmunohistoquímica en dermatopatología: revisión de los anticuerpos utilizados con mayor frecuencia (parte ii). Actas Dermosifiliogr. 2013;104:181–203.