This past year has seen a wealth of new developments in dermatopathology that appear to herald the dawning of a new era. Advances in molecular biology and the simplification of techniques have put molecular tests within reach of routine clinical practice and led to a radical change in our approach to lesions such as melanoma; in the future, the genetic characterization of these lesions will be an essential requirement for establishing diagnosis, prognosis, and therapy. Technological innovations have also reached dermatology departments: the introduction of ultrasound scans has propitiated the use of fine-needle aspiration cytology, which allows samples to be stained and studied immediately, thereby facilitating diagnosis of superficial and lymph-node lesions, and allowing staging of tumors such as melanoma. Targeted cancer therapies have led to the introduction of more sensitive and specific systems for identifying new targets, have reawakened interest in forgotten diseases such as aggressive basal cell carcinoma, and have led to dermatological reactions that, together with those caused by biologic drugs, we are just beginning to recognize. Consolidated techniques such as immunohistochemistry continue to advance with the addition of new antibodies that contribute considerably to improved diagnosis. New clinicopathologic diseases have also been described or characterized this year, including 2 new types of melanoma, and progress has been made in our knowledge of other diseases, such as primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma. These topics, together with new developments in adnexal tumors, alopecia, and other lesions, will be discussed in this review.
Este último año ha sido rico en novedades dermatopatológicas que se vislumbran como el inicio de una nueva era. Los avances en las técnicas de biología molecular y su simplificación, que las hace asequibles al uso rutinario, han supuesto un cambio radical en el concepto de lesiones como el melanoma, cuya caracterización genética será en el futuro un requisito indispensable para establecer el diagnóstico, el pronóstico y la terapéutica. Las innovaciones tecnológicas también han llegado a los servicios de Dermatología; la introducción de ecógrafos ha propiciado el uso de técnicas de citología por punción-aspiración que pueden ser teñidas y estudiadas de manera inmediata, facilitando el diagnóstico de lesiones superficiales y ganglionares, y permitiendo la estadificación de neoplasias como el melanoma. La aparición de tratamientos oncológicos dirigidos contra «dianas terapéuticas» ha conllevado la introducción de sistemas más sensibles y específicos para su idetificación, ha reactivado el interés por patologías olvidadas como el carcinoma basocelular agresivo y ha comportado la aparición de reacciones dermatológicas que, junto con las secundarias a fármacos biológicos, empezamos a reconocer. Técnicas consolidadas como la inmunohistoquímica continúan progresando con la incorporación de nuevos anticuerpos que contribuyen de manera notable al diagnóstico. Este año, además, se han descrito o caracterizado nuevas entidades clinicopatológicas, y entre ellas 2 nuevos tipos de melanoma. También se ha avanzado en el conocimiento de otras, como el linfoma T pleomórfico CD4+ de células pequeñas/medianas. Estos temas, junto con novedades en tumores anexiales, alopecias y otras lesiones, serán comentados en esta revisión.
The past year has seen a wealth of new developments in dermatopathology, many of which are related to technical progress and advances in treatment. This short review covers some of the developments that may have the greatest impact on the pathological diagnosis of dermatological disorders in the following 6 main areas: melanocytic proliferations, inflammatory diseases (including drug-induced reactions), lymphoproliferative disorders, adnexal diseases, epidermal tumors, and mesenchymal tumors.
Melanocytic ProliferationsMelanoma has been one of the prominent topics in dermatopathology in recent years. The recent introduction of fluorescent in situ hybridization as a ancillary diagnostic test in the identification of malignant melanocytic tumors was a major advance in an area that had languished for decades.1 Unfortunately, the evidence shows that commercially available diagnostic kits providing a set of probes targeting chromosomes 6 and 11 are inadequate in problematic tumors.2–4 The sensitivity and specificity obtained in ambiguous tumors is lower than expected. To optimize the results obtained, new probes should be added to the diagnostic kit, possibly targeting gene regions on chromosome 9. However, it is increasingly clear that the genetic aberrations present in each melanoma correlate with the tumor's clinical and pathologic features and biological behavior,5,6 and that they can help to elucidate the considerable differences in the clinical course of melanomas that are apparently in the same stage.
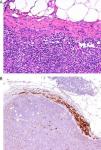
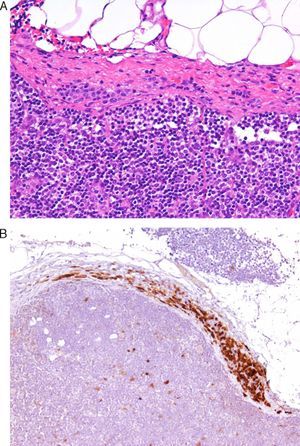
Today, from a practical standpoint, the most accessible and manageable techniques in most pathology laboratories are immunohistochemical studies. Thus, studies that demonstrate the usefulness of immunohistochemical markers are of particular interest. One such example is a study showing CD10 expression to be an independent predictor of poor prognosis in melanoma.7 Several studies have shown an association between the loss of immunohistochemical p16 expression and malignant transformation in melanocytic lesions. This finding may be a useful aid in the differentiation between Spitz nevus and childhood melanoma,8 and between nodal nevi in sentinel lymph nodes and small melanoma metastases (Fig. 1).9 The usefulness of p16 as a marker is less clear in other lesions, such as pigmented spindle cell nevi.4 Furthermore, a loss of p16 expression is typically observed in patients with dysplastic nevi, in which its absence is an initial step in the development of melanoma.10
A, The presence of small intracapsular nodal nevi in the sentinel lymph nodes of a melanoma is not uncommon, and this phenomenon can cause diagnostic difficulties when the primary tumor is a nevoid melanoma. (Hematoxylin-eosin, original magnification x200.) B, The preservation of p16 expression could be an additional indication of a benign neoplasm. (p16, original magnification x200.).
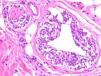
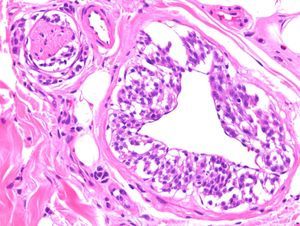
Two studies provided reassuring evidence concerning the low risk of metastases in Spitzoid lesions and atypical melanocytic proliferations in childhood.11,12 Angiotropism may explain why these lesions spread to sentinel lymph nodes but lack the potential to generate disseminated metastases: melanocytes may spread to the lymph nodes through the perivascular space, but lack the capacity to invade the lymphatic system or the bloodstream. The phenomenon, which is similar to perineural infiltration, is also observed in some melanomas (Fig. 2.) Cells that migrate to lymph nodes by means of this mechanism cannot strictly speaking be considered metastases. A group of highly regarded dermatopathologists advocate the use of the vascular markers D2-40 and CD34 to clearly define the relationship between tumor cells and vascular structures.13
Melanoma demonstrating angiotropism and neurotropism. Angiotropism, a phenomenon analogous to neurotropism, is found in both benign and malignant melanocytic tumors. It could explain how melanocytes spread to lymph nodes without being true metastases. (Hematoxylin-eosin, original magnification x200.).
The introduction of ultrasound scanners into dermatology departments has opened the door to the possibility of using fine-needle aspiration biopsies to investigate superficial tumors and suspected metastatic lymph nodes (a practice that does not have to be restricted to interventional radiologists or pathologists; by analogy, some endocrinologists perform fine-needle aspiration biopsies of the thyroid). Some authors have proposed using fine-needle aspiration cytology to identify metastatic melanoma in sentinel nodes and suspicious lymph nodes.14,15 They report that a negative result on ultrasound study for a sentinel node is very reliable, and when metastasis is suspected, immediate cytologic diagnosis can be obtained using ultrasound-guided fine-needle aspiration biopsy. If the cytology is positive for metastasis, lymphadenectomy can be performed without further investigation, thus reducing morbidity and expenditure. Sonomorphologically suspicious nodes in which the presence of metastases is not detected by cytology should undergo complete study according to the usual protocols for sentinel nodes.
Two new histopathologic variants of melanoma have been described this year. The first of these is a variant of superficial spreading melanoma characterized by large junctional melanocytic nests.16 This variant may go undetected on histopathology study because it lacks many of the well-known microscopic diagnostic criteria of malignancy. It usually develops on areas of the skin with actinic damage and, although growth tends to be radial, it can infiltrate the dermis. The second, which is associated with inactivating germline mutations of BAP1 (BRCA1 associated protein-1),0,17 is a new hereditary syndrome that predisposes patients to epithelioid melanocytic nevi, atypical Spitzoid proliferation, cutaneous and uveal melanoma, lung cancer, meningioma, mesothelioma, and other cancers.18 Moreover, individuals who do not have this hereditary predisposition can also present atypical epithelioid melanocytic proliferations that harbor inactivating mutations of BAP1 and concomitant BRAF mutations (V-raf murine sarcoma viral oncogene homolog B1).19 Lesions associated with inactivation of BAP1 are characterized by a lack of correlation between their microscopic appearance resembling an atypical Spitz nevi and a relatively unremarkable clinical appearance, similar to an Unna or Miescher nevus.
Finally, the introduction of targeted treatment for metastatic melanomas that test positive for the BRAF mutation has led to the development of automated techniques for detecting this target; these new techniques are more sensitive than traditional polymerase chain reaction followed by sequencing. In line with the widespread and growing practice of creating alliances between the companies that market biomarker detection techniques and those that produce the drugs targeting those molecules, the company that markets a drug that inhibits the B-Raf protein also distributes the device that detects the mutation that determines its therapeutic indication. This device offers greater sensitivity and specificity than conventional sequencing techniques or polymerase chain reaction.
Drug-Induced Reactions and Other Inflammatory ProcessesThe introduction of new drugs often gives rise to the appearance of skin reactions with previously unknown histopathologic patterns, and the reactions to treatments targeting biomarkers are a good example of this phenomenon. In patients treated with vemurafenib—a B-Raf inhibitor indicated in melanomas with V600E mutations—various skin reactions have been described, including a lobular neutrophilic panniculitis20 that is often painful and associated with arthritis; in such cases the microscopic changes are focal and sometimes very subtle. The lesions most often biopsied in patients receiving this treatment are wart-like proliferations with features that fall between common viral warts, keratoacanthomas, and squamous cell carcinomas (Fig. 3).21 Patients receiving treatment with sorafenib,22 which has been prescribed for metastatic melanoma harboring KIT mutations, among other indications, also develop epithelial proliferations that are not usually papillomatous but tend to form cysts and are frequently related to hair follicles. Sorafenib also produces manifestations similar to those of pityriasis rubra pilaris.23
Biologic therapies, first introduced some years ago, have also led to the emergence of several reactions that have now begun to be well characterized. A recent review of the adverse effects of tumor-necrosis-factor (TNF) antagonists included erythema multiforme-like and lupus erythematosus-like lesions in addition to the neutrophil-rich disorders (psoriasiform dermatitis, palmoplantar pustular psoriasis, pustular folliculitis, Sweet syndrome, neutrophilic eccrine hidradenitis, and leukocytoclastic vasculitis).24 In another article, TNF antagonists were reported to have caused a pseudolymphomatous drug reaction.25 Such reactions have also been reported in patients receiving ustekinumab.26
Continuing in the field of drug reactions, one recently published article refutes the idea that eosinophilia in subacute lupus erythematosus is an indicator of a drug reaction,27 and another reviews a series of cases in which the initial clinical and pathological diagnosis of drug-induced reaction was subsequently proven to be inaccurate.28 This last study suggests that the label of drug-induced reaction is overused in such cases, especially in the case of maculopapular eruptions.
A recent study29 called into question the prognostic value of the severity of inflammatory dermal infiltrate in toxic epidermal necrolysis.30 The severity of dermal infiltrate had previously been considered to be directly correlated with the risk of death in the course of this disease. In that study, only the depth of the epidermal necrosis was shown to have prognostic significance, and even that was not an independent predictor.29
In severe leukocytoclastic vasculitis, exocytosis of neutrophils to the surface can lead to the appearance of microabscesses on the tips of papillae, mimicking dermatitis herpetiformis. The publication of a case of dermatitis herpetiformis that began with petechiae and leukocytoclastic vasculitis alerts us to the possibility of making the opposite mistake.31 Four new cases of—mostly systemic—diffuse nonbullous neutrophilic lupus erythematosus were reported during the year, further defining a newly emerging entity that is very difficult to recognize microscopically.32
A rather curious study on biopsy samples destined for immunofluorescence testing examined the effect of accidental immersion of the samples in formalin before freezing.33 Unsurprisingly, the study revealed that even 2minutes of immersion in formalin led to complete loss of the staining characteristics in cases of pemphigus and under 10minutes in those of pemphigoid. The most fascinating finding was that immunologic deposits in the dermatitis herpetiformis samples resisted up to 2hours of immersion in formalin. It is also of note that, after prolonged immersion in formalin, keratinocyte nuclei acquire autofluorescence, thereby simulating lupus erythematosus.
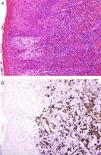
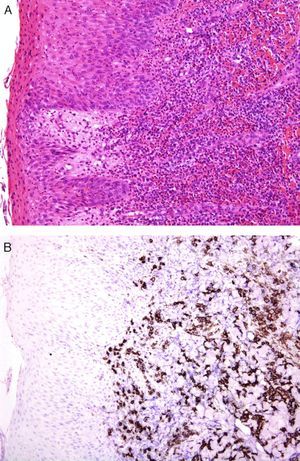
An international group of European dermatopathologists has collected and studied 13 cases of lesions that had the clinical appearance and behavior of acute pityriasis lichenoides, but in which the biopsy results included T-cell clonality and a large number of CD30+ cells, which often coexpressed CD8 (Fig. 4).34 Not only do these lesions microscopically simulate lymphomatoid papulosis, they may, in fact, belong to the same spectrum as this disorder. The same authors also found evidence of parvovirus 19 DNA in 40% of the cases, a finding which, together with the immunophenotype consisting of CD8+ and CD30+ cells, suggests a possible viral etiology.34
A, Lichenoid eruption in a child. The lesion has the clinical features of pityriasis lichenoides et varioliformis acuta but demonstrates lymphocytic atypia. (Hematoxylin-eosin, original magnification x200.) B, The lesion contains abundant CD30+ lymphoid cells. (CD30, original magnification x200.).
Also described recently was a new pseudolymphomatous variant of granuloma annulare rich in CD8+ T cells, a new variant that has been added to the list of known forms of this disease (necrobiotic, incomplete or interstitial, and the rare sarcoid and tuberculoid forms).35
Also on the subject of pseudolymphomatous lesions, a case of lipoatrophic panniculitis with the microscopic appearance of panniculitis-like T-cell lymphoma was reported in a pediatric patient; the pathogenesis was probably autoimmune.36 Other forms of panniculitis that may pose problems in the differential diagnostic are those caused by cutaneous and mucosal mucormycosis, which can convincingly mimic pancreatic or gouty panniculitis; this similarity may, in part, be explained by the ability of these fungi to produce extracellular lipase.37
Lymphoproliferative DisordersThe most interesting advance in the field of lymphomas has been the characterization of primary cutaneous CD4+ small-to-medium-sized pleomorphic T-cell lymphoma, a provisional entity in the World Health Organization (WHO) classification. Owing to the low aggressiveness of these tumors, especially in the case of single-lesion disease, they are considered to represent a clonal T-cell lymphoproliferative disorder with an indolent clinical course. This entity is characterized by a proliferation of follicular helper T cells characterized by the expression of PD-1 (programmed cell death 1), a feature that can be used to distinguish it from other types of cutaneous T-cell lymphomas.38
It is possible that the cases currently included in this provisional entity can be divided into 2 categories. Most of these patients present a low-grade lymphoproliferative disorder analogous to a pseudo-T-cell lymphoma (usually solitary lesions),38 while a small number of them present a follicular helper T-cell lymphoma (usually multiple lesions), which has a much more aggressive biological behavior and requires intense treatment.39 In these lymphomas, the presence of numerous B lymphocytes in the infiltrate and the expression of CD10 in the neoplastic lymphocytes may give rise to confusion with follicular lymphoma.
Adnexal DiseasesTwo cases have recently been described of eruptive hyperplasia of ectopic sebaceous glands after episodes of epidermal erosion caused by scald in one case40 and following Stevens-Johnson syndrome in the other.41
In the field of alopecia, 2 new diagnostic clues have been reported.42,43 The first report highlights the utility of polarized light microscopy to distinguish between the scars of scarring alopecia (which are polarizable) and the fibrous streamers of androgenic alopecia which, despite having a very similar microscopic appearance, do not polarize.42 The second article, by the same authors, provides a clue to the diagnosis of frontal fibrosing alopecia, which the authors have called the follicular triad.43 The term refers to the simultaneous involvement of follicles of 3 types (terminal, vellus, and intermediate) in all 3 stages of cycling (anagen, catagen, and telogen).43 This sign can be very useful in identifying this variant of lichenoid alopecia, which is not restricted to the frontal region, but can affect the entire scalp and other areas of the skin. Another symptom that has recently been added is facial papules secondary to the involvement of facial vellus hair.44
Still on the subject of alopecia, 2 independent studies show that permanent alopecia caused by systemic chemotherapy has histologic features identical to those of androgenic alopecia, leading to diagnostic difficulties.45,46 Finally, a new way has been proposed to optimize the yield of a single punch biopsy using a technique that produces both horizontal sections, which are very useful in the diagnosis of alopecia, and vertical sections, which allow us to assess the involvement of the epidermis and the uppermost layer of the dermis, also a source of relevant information.47 The technique involves transecting the biopsy approximately 1mm below the skin surface and using this to obtain vertical sections (perpendicular to the epidermis) and then sectioning the lower portion to obtain horizontal sections (parallel to the epidermis).
Moving on to the subject of adnexal tumors, one of the most striking items that appeared in the last year was the description of desmoplastic trichoepithelioma with perineural involvement, an extremely unusual feature in benign tumors but one that can no longer be considered a reliable sign of malignancy in this context.48 Eosinophilia has also been proposed as a clue to the diagnosis of microcystic adnexal carcinoma.49
Epidermal TumorsThe introduction of hedgehog pathway inhibitors for the treatment of advanced basal cell carcinoma50 has stimulated the publication of numerous reports on cases of aggressive basal cell carcinoma.51,52 It is unclear why some of these tumors have such a high malignant potential that they grow to a large size, invade deep tissues, and even produce metastases. In 1 review of the literature,51 basosquamous cell carcinoma is cited as one of the most aggressive variants of this type of tumor and the one that most often metastasizes; however in another study the same variant, which is considered rare, is said to have a similar prognosis to typical basal cell carcinoma even in transplant recipients.53 In any case, the authors of the latter advocate the use of the Ber-EP4 antibody (Clone Ber-EP4) to confirm the diagnosis of this tumor; this marker is positive in areas of basal cells and negative in those of squamous cells.53 A group of Spanish authors have also made an interesting discovery with respect to Ber-EP4, observing that it stained both sides of the retraction spaces surrounding epithelial nests in basal cell carcinoma.54 Ber-EP4, also known as EpCAM (epithelial cell adhesion molecule), is directed against a specific epithelial antigen (a surface glycoprotein), so that staining on the exterior aspect of the retraction spaces would appear to indicate that these are epithelial in nature. In short, the retraction spaces typical of basal cell carcinoma are not, as has always been thought, located between the epithelium and the stroma, but rather occupy a more peripheral portion of the epithelium.54
Finally, several articles have appeared confirming the predictive value of the new American Joint Committee on Cancer staging system for squamous cell carcinoma,55 which includes indicators of poor prognosis not included in the TNM cancer staging system, such as poor histologic differentiation, thickness greater than 2mm, a Clark level of IV or more, the presence of vascular and/or perineural invasion, and location on the ear or lip.
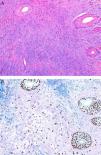
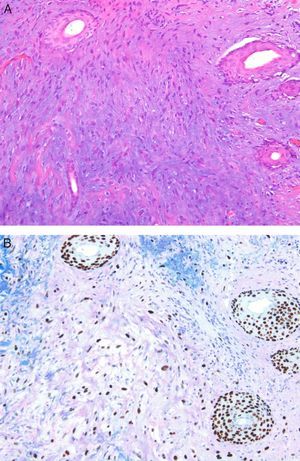
Mesenchymal TumorsA recent article confirmed that the expression of p63 is rare in mesenchymal tumors, and when it occurs is usually focal or weak, confirming the usefulness of this protein in identifying pseudosarcomatous carcinomas (Fig. 5).56 Similarly, new studies have confirmed that the amplification of MYC (avian myelocytomatosis virus) is an excellent marker for the cutaneous angiosarcomas that appear after radiotherapy or in areas of lymphedema, distinguishing them from other postradiation malignancies and angiosarcomas unrelated to radiotherapy. Moreover, immunohistochemical overexpression is as reliable as fluorescent in situ hybridization for the detection of this marker.57,58
A, Dermis replaced by dense collagen surrounding spindle cells with a mesenchymal appearance. (Hematoxylin-eosin, original magnification x100.) B, p63 positivity demonstrates the epithelial origin and facilitates the diagnosis of spindle cell squamous cell carcinoma. (p63, original magnification x100.).
Finally, the term pleomorphic dermal sarcoma has been proposed to denominate lesions that resemble atypical fibroxanthomas microscopically but produce deep tissue invasion, extensive necrosis, and/or lymphovascular or perineural invasion.59 We have even had room for controversy in this area, such as that arising from the description of pseudomyogenic hemangioendothelioma, previously called epithelioid sarcoma-like hemangioendothelioma.60
ConclusionIn conclusion, in a year full of new developments, significant advances have been made in the diagnosis, staging, and treatment of melanocytic lesions. New drug-induced reactions have been described, especially reactions to the drugs more recently introduced into the therapeutic arsenal. Important advances have been made in the characterization of primary cutaneous CD4+ small-to-medium-sized pleomorphic T-cell lymphoma, a provisional WHO entity that, in many cases, is consistent with T pseudolymphomas. Likewise, we have seen the publication of new diagnostic features for alopecia and reports on the presence of perineural invasion in desmoplastic trichoblastoma. In addition, there has been confirmation of the usefulness of p63 and MYC in the identification of pseudosarcomatous epithelial proliferations and angiosarcomas secondary to radiotherapy or arising on lymphedema. Likewise, it has been confirmed that there is an excellent correlation between the new American Joint Committee on Cancer staging system for squamous cell carcinoma of skin and the biological behavior of these lesions.
These and other excellent advances which, for reasons of space, could not be included in this brief review, are a reflection of the brilliant scientific production that continuously enriches the field of dermatopathology and allows us to be very optimistic about the future of this discipline.
Ethical DisclosuresProtection of Human and Animal SubjectsThe authors state that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that they have followed the protocols of their workplace concerning the publication of patient data, and that all the patients included in this study have been appropriately informed and gave their written informed consent to participate in this study.
Right to Privacy and Informed ConsentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-Figueras MT, Puig L. Actualización en dermatopatología. Actas Dermosifiliogr. 2013;104:204–11.