Dermatofibrosarcoma protuberans (DFSP) is a rare, slow-growing cutaneous tumor that can invade the subcutaneous tissue, muscle tissue, and even bone.
ObjectiveTo identify histologic features associated with greater depth of invasion, i.e., local aggressiveness, in DFSP.
Material and methodsWe analyzed associations between histologic features of DFSP (e.g., type of subcutaneous invasion, histologic pattern, cell type, areas of fibrosarcoma) and the presence and absence of muscle fascia involvement.
ResultsWe studied 155 cases of DFSP. The following histologic characteristics were significantly associated with involvement of the muscle fascia: the presence of a sheetlike pattern, a high degree of cellular pleomorphism, and more than 1 mitotic figure. The tumor did not extend beyond the subcutaneous tissue in the majority of cases (62.6%), but there was involvement of the fascia or galea aponeurotica in 17 cases (11%) and of the muscle tissue in 36 cases (23.2%).
ConclusionsHistologic patterns, degree of pleomorphism, and number of mitotic figures are important predictors of deep invasion (fascia or muscle) in DFSP; these layers can be involved in up to 30% of cases.
El dermatofibrosarcoma protuberans (DFSP) es un raro tumor cutáneo de crecimiento lento e infiltrativo que alcanza el tejido celular subcutáneo, el tejido muscular e incluso el hueso.
ObjetivosBuscar las características histológicas asociadas a una mayor agresividad local en los DFSP, en forma de afectación en profundidad.
Material y métodosSe relacionó las características histológicas propias del DFSP (forma de infiltrar el tejido celular subcutáneo, patrón histológico, tipo celular, áreas de fibrosarcoma) con la presencia o ausencia de afectación de la fascia muscular.
ResultadosSe incluyeron 155casos de DFSP. Las características histológicas asociadas significativamente con la afectación de la fascia muscular fueron: el patrón histológico en sábana, un alto grado de pleomorfismo celular y la presencia de más de una mitosis. En la mayoría de los casos (62,6%) el tumor se limitó al tejido celular subcutáneo, en 17 casos (11%) contactó con la fascia muscular o con la galea aponeurótica, y en 36 casos (23,2%) afectó al tejido muscular.
ConclusionesEs importante tener en cuenta el patrón histológico, el pleomorfismo y el número de mitosis en los DFSP para predecir su afectación en profundidad (fascia o músculo), que puede llegar al 30% de los casos.
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, slow-growing,invasive fibrohistiocytic tumor with low metastatic potential and high local recurrence.1 The translocation of genetic material between the platelet-derived growth factor subunit B gene (PDGFB) and the collagen type 1 alpha 1 gene (COL1A1) gives rise to the COL1A1-PDGFB fusion gene, which is specific to DFSP and has an important pathogenic role in this tumor.2 DFSP initially presents as a small brown, reddish-brown, or flesh-colored plaque. In its early stages, it can go unnoticed or be confused with a benign lesion. It grows slowly, but with time, it extends both vertically and horizontally and forms protruding nodules on the surface, hence the term protuberans.3
Microscopically, DFSP has the appearance of a well-differentiated fibrosarcoma. It is formed by a dense proliferation of uniform spindle cells with a large elongated nucleus, minimal pleomorphism, and low mitotic activity.4 The stroma contains varying amounts of collagen and capillary vessels. One of the main histologic characteristics of DFSP, and one it shares with other fibrohistiocytic tumors, is the presence of irregularly interlaced fascicles of spindle cells forming a storiform pattern.5 The cells are more tightly packed together in the center part of the tumor than at its edges, which feature finger-like projections consisting of fibrous tracts with few cells that invade the subcutaneous tissue, the muscle fascia, and even the bone.6 These tentacle-like projections can reach great distances from the center of the tumor. The extent of subclinical disease is therefore highly unpredictable and can be difficult to detect by conventional histology, resulting in high recurrence, even in cases of surgical excision with wide margins.7,8 The main histologic feature of DFSP is the pattern of subcutaneous infiltration.9 DFSP typically invades the subcutaneous tissue through the septa and even the fat lobules, forming the well-known honeycomb pattern or parallel-band pattern, which consists of multiple layers of spindle cells, intermixed with uninvolved layers of fat, oriented parallel to the skin surface. Less frequently, the tumor can infiltrate downwards through the septa, forming a digitiform pattern in the subcutaneous fat and/or a club-like compressive pattern.5 Several histologic variants have been described within these tumors, albeit with varying frequency. These variants include giant cell fibroblastoma, pigmented DFSP (Bednar tumor), atrophic DFSP, sclerotic DFSP, myoid DFSP, myxoid DFSP, and fibrosarcomatous DFSP.5
Complete surgical excision is the treatment of choice for DFSP,10 but it should be recalled that the eccentric growth of the tumor, via fingerlike projections, means that DFSP is highly asymmetric and can extend far from the center of the tumor. These tentacle-like projections that emerge from the edges of the tumor explain the high rates of local recurrence observed in DFSP.5 Mohs micrographic surgery (MMS) is the treatment of choice for DFSP, as it provides a complete histologic study of all the surgical margins.11
The fact that DFSP largely follows a favorable course and has low metastatic potential perhaps explains why no truly rigorous studies have analyzed the prognostic factors associated with this tumor. While mortality is generally low in DFSP, morbidity can be high in more aggressive tumors or in tumors located in areas where complete surgical excision is difficult. The most challenging tumors from a surgical perspective are large tumors, long-standing tumors, deep-seated infiltrative tumors, and tumors located in the head and neck region. The only histologic factor associated to date with increased local aggressiveness, however, is the presence of fibrosarcomatous areas.12
The main aim of this study was to determine which histologic factors are associated with greater subclinical extension and greater local aggressiveness in DFSP. Our secondary aims were to determine the depth of invasion in a large series of DFSP to quantify local aggressiveness and to perform a descriptive study of the histologic characteristics of this skin tumor.
Material and MethodsWe performed a retrospective observational study of DFSP cases seen at our department over a period of 13 years (1998-2011). Only cases with histologic slides representative of the tumor or with paraffin-embedded specimens containing sufficient tumor tissue for creating and staining new slides were included.
We evaluated the following histologic data:
- -
Predominant histologic pattern
- 1.
Storiform pattern: fascicles of dense cells with a whorled, wave-like arrangement
- 2.
Cartwheel pattern: fascicles of cells radiating from a central axis
- 3.
Sheetlike pattern: uniformly arranged cells forming neither clusters nor fascicles
- 4.
Fascicular pattern: cells arranged in fascicles along a long central axis, similar to a herringbone
- 1.
- -
Pattern of subcutaneous tissue infiltration
- 1.
Honeycomb pattern: cells distributed among the septa and fat lobules, forming a honeycomb or lacelike pattern
- 2.
Digitiform: cells distributed vertically or radially through the septa
- 3.
Parallel-band pattern: cells arranged predominantly in multiple layers parallel to the skin surface
- 4.
Compressive pattern: cells arranged in a well-delimited, club-like pattern
- 1.
- -
Cell density: number of cells in a representative area of the tumor measuring 2000μm2 at ×40 magnification
- -
Moderate: <100 cells
- -
High: 100-150 cells
- -
Very high: >150 cells
- -
- -
Predominant cell type: epithelioid or spindle
- -
Degree of cellular pleomorphism: none, minimal, moderate, or high
- -
Number of mitotic fields per 10 fields of view at x40 magnification
- -
Presence of areas of fibrosarcoma: >5% of the tumor surface with cells arranged in fascicles along a longitudinal axis (herringbone pattern) or >5% highly pleomorphic cells with many mitotic figures, with no specific arrangement
- -
Presence of other histologic variants of DFSP: pigmented (Bednar tumor), giant cell fibroblastoma, myxoid, myoid, or sclerotic
- -
Deep histologic invasion (deepest involved layer): subcutaneous tissue, muscle fascia, muscle, periosteum, or bone
The modified MMS technique, known as slow Mohs,13 was used to treat all the tumors included in this study. To determine the exact depth of invasion, serial sections were obtained from the debulking specimen—or from the scar tissue of excised tumors with positive margins—and stained with hematoxylin and eosin. In several cases, multiple serial slices were examined to identify or rule out the presence of tumor foci.
Local aggressiveness was defined as involvement of the muscle fascia (or the galea aponeurotica in tumors of the scalp). We analyzed the association between the presence or absence of local aggressiveness and each of histologic features evaluated. For this part of the study, we included only primary tumors to ensure a more faithful reproduction of our results in future studies, as there is a greater risk of including a poorly representative part of the tumor in recurrent tumors or excised tumors with histologically positive margins.
SPSS was used for statistical analyses. The distribution of independent variables in relation to the dependent variable (invasion of the fascia or galea aponeurotica) was evaluated using the Pearson χ2 test or the Fisher exact test for expected values of under 5%. Statistical significance was set at a level of P<.05.
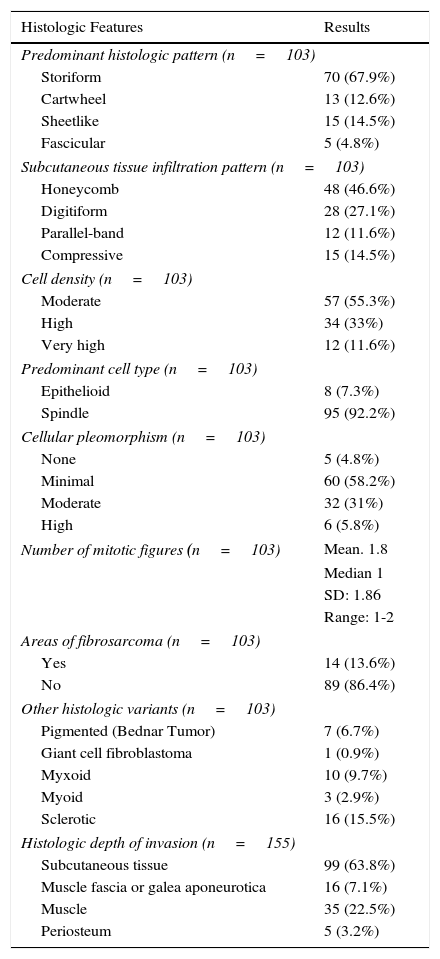
ResultsWe included 155 cases of DFSP in which it was possible to determine the depth of invasion. Of these, 103 had sufficient tumor material for the histologic study; 73 were primary tumors, 22 were recurrent tumors, and 8 were tumors that had been removed with positive margins. The results of the histologic study are shown in Table 1.
Histologic Features of Our Series of Dermatofibrosarcoma Protuberans.
| Histologic Features | Results |
|---|---|
| Predominant histologic pattern (n=103) | |
| Storiform | 70 (67.9%) |
| Cartwheel | 13 (12.6%) |
| Sheetlike | 15 (14.5%) |
| Fascicular | 5 (4.8%) |
| Subcutaneous tissue infiltration pattern (n=103) | |
| Honeycomb | 48 (46.6%) |
| Digitiform | 28 (27.1%) |
| Parallel-band | 12 (11.6%) |
| Compressive | 15 (14.5%) |
| Cell density (n=103) | |
| Moderate | 57 (55.3%) |
| High | 34 (33%) |
| Very high | 12 (11.6%) |
| Predominant cell type (n=103) | |
| Epithelioid | 8 (7.3%) |
| Spindle | 95 (92.2%) |
| Cellular pleomorphism (n=103) | |
| None | 5 (4.8%) |
| Minimal | 60 (58.2%) |
| Moderate | 32 (31%) |
| High | 6 (5.8%) |
| Number of mitotic figures (n=103) | Mean. 1.8 |
| Median 1 | |
| SD: 1.86 | |
| Range: 1-2 | |
| Areas of fibrosarcoma (n=103) | |
| Yes | 14 (13.6%) |
| No | 89 (86.4%) |
| Other histologic variants (n=103) | |
| Pigmented (Bednar Tumor) | 7 (6.7%) |
| Giant cell fibroblastoma | 1 (0.9%) |
| Myxoid | 10 (9.7%) |
| Myoid | 3 (2.9%) |
| Sclerotic | 16 (15.5%) |
| Histologic depth of invasion (n=155) | |
| Subcutaneous tissue | 99 (63.8%) |
| Muscle fascia or galea aponeurotica | 16 (7.1%) |
| Muscle | 35 (22.5%) |
| Periosteum | 5 (3.2%) |
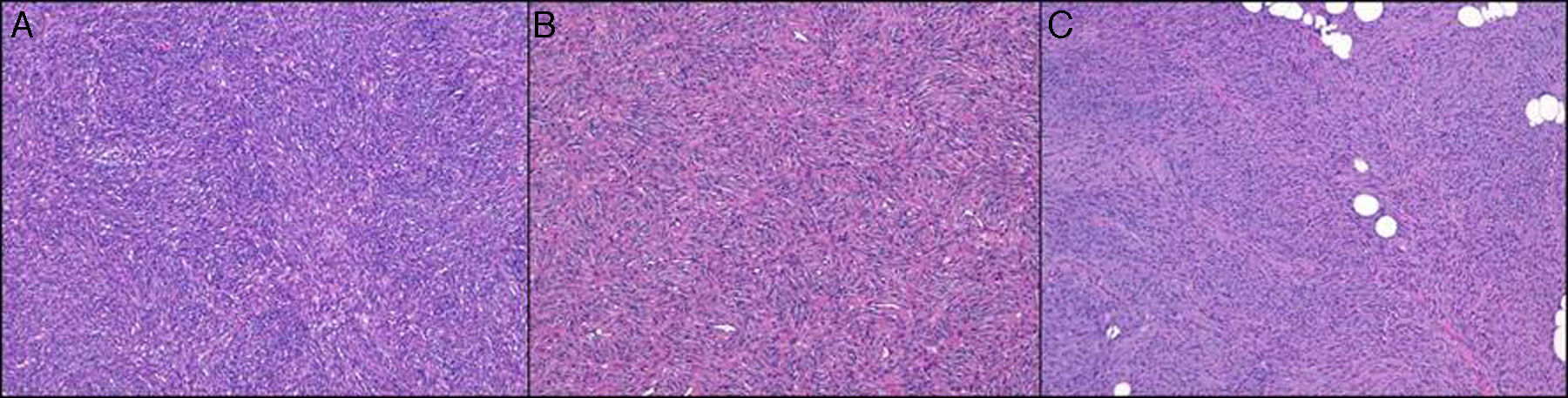
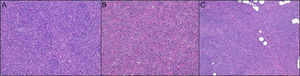
The predominant histologic pattern was the storiform pattern (67.9% of cases), while the predominant subcutaneous infiltration pattern was the honeycomb pattern (46.6%) followed by the digitiform pattern (27.1%) (Fig. 1).
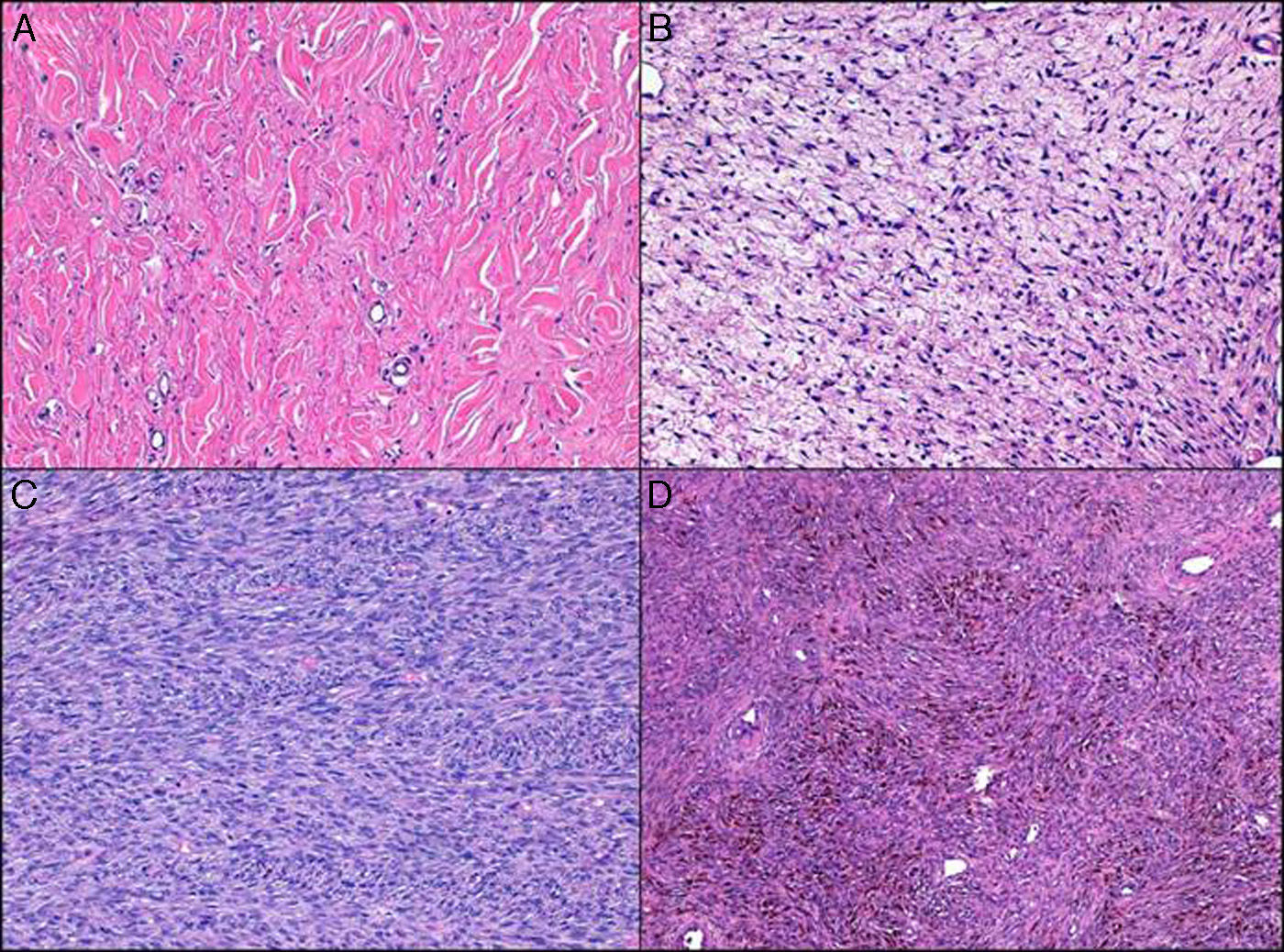
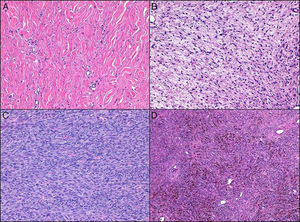
Cell density was moderate in over half of the cases (55.3%), and the predominant cell type was spindle (92.2%). Of note were the low degree of pleomorphism observed (none in 4.8% of cases and minimal in 58.2%) and the small number of mitotic figures (mean of 1.8 and median of 1). The following histologic variants were identified: areas of fibrosarcoma (14 cases, 13.6%), sclerotic DFSP (16 cases, 15.5%), myxoid DFSP (10 cases, 9.7%), pigmented DFSP (7 cases, 6.7%), and giant cell fibroblastoma (1 case) (Fig. 2).
In most cases (62.6%), the tumor did not extend beyond the subcutaneous tissue; there was involvement of the fascia or galea aponeurotica in 17 cases (11%) and of the muscle tissue in 36 cases (23.2%). The periosteum was affected in just 5 cases (3.2%).
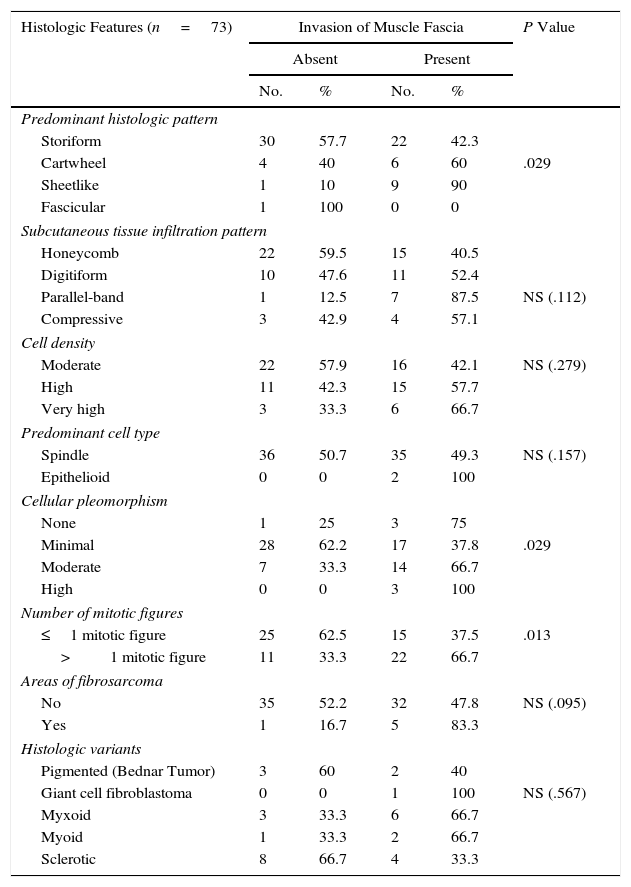
Regarding the association between depth of invasion and the different histologic features analyzed (Table 2), we observed a high degree of cellular pleomorphism in the sheetlike and cartwheel patterns and a significant association between the presence of more than 1 mitotic figure and involvement of the muscle fascia or galea aponeurotica. None of the other histologic features were significantly associated with local aggressiveness.
Association Between Histologic Features and Involvement of the Muscle Fascia or Galea Aponeurotica in Primary Dermatofibrosarcoma Protuberans.
| Histologic Features (n=73) | Invasion of Muscle Fascia | P Value | |||
|---|---|---|---|---|---|
| Absent | Present | ||||
| No. | % | No. | % | ||
| Predominant histologic pattern | |||||
| Storiform | 30 | 57.7 | 22 | 42.3 | |
| Cartwheel | 4 | 40 | 6 | 60 | .029 |
| Sheetlike | 1 | 10 | 9 | 90 | |
| Fascicular | 1 | 100 | 0 | 0 | |
| Subcutaneous tissue infiltration pattern | |||||
| Honeycomb | 22 | 59.5 | 15 | 40.5 | |
| Digitiform | 10 | 47.6 | 11 | 52.4 | |
| Parallel-band | 1 | 12.5 | 7 | 87.5 | NS (.112) |
| Compressive | 3 | 42.9 | 4 | 57.1 | |
| Cell density | |||||
| Moderate | 22 | 57.9 | 16 | 42.1 | NS (.279) |
| High | 11 | 42.3 | 15 | 57.7 | |
| Very high | 3 | 33.3 | 6 | 66.7 | |
| Predominant cell type | |||||
| Spindle | 36 | 50.7 | 35 | 49.3 | NS (.157) |
| Epithelioid | 0 | 0 | 2 | 100 | |
| Cellular pleomorphism | |||||
| None | 1 | 25 | 3 | 75 | |
| Minimal | 28 | 62.2 | 17 | 37.8 | .029 |
| Moderate | 7 | 33.3 | 14 | 66.7 | |
| High | 0 | 0 | 3 | 100 | |
| Number of mitotic figures | |||||
| ≤1 mitotic figure | 25 | 62.5 | 15 | 37.5 | .013 |
| >1 mitotic figure | 11 | 33.3 | 22 | 66.7 | |
| Areas of fibrosarcoma | |||||
| No | 35 | 52.2 | 32 | 47.8 | NS (.095) |
| Yes | 1 | 16.7 | 5 | 83.3 | |
| Histologic variants | |||||
| Pigmented (Bednar Tumor) | 3 | 60 | 2 | 40 | |
| Giant cell fibroblastoma | 0 | 0 | 1 | 100 | NS (.567) |
| Myxoid | 3 | 33.3 | 6 | 66.7 | |
| Myoid | 1 | 33.3 | 2 | 66.7 | |
| Sclerotic | 8 | 66.7 | 4 | 33.3 | |
Abbreviation: NS, not significant.
Histologically, DFSP typically consists of a dense proliferation of spindle cells with scant cytoplasm that form a storiform pattern, have a uniform appearance, and exhibit minimal pleomorphism.1 Our findings are consistent with this pattern. Of potential interest in our series is the moderate cell density observed in the majority of tumors. This may partly be explained by the fact that we defined moderate cell density as fewer than 100 cells in a representative section of the tumor viewed at ×40 magnification. In addition, in most cases the surface of the tumor occupied by tumor cells was larger than that occupied by collagenous stroma.
The arrangement of cells in short, interlacing fascicles, forming the storiform pattern, is typical of fibrohistiocytic tumors and particularly specific to DFSP. Although this pattern is common, other patterns, such as the cartwheel, sheetlike, and fascicular patterns, are occasionally seen, with varying predominance. This was the case in our series, in which the storiform pattern was the most common pattern observed (67.9% of cases), followed by the sheetlike pattern (14.5%) and the cartwheel pattern (12.6%). The cartwheel pattern may simply be a variant of the storiform pattern with particularly short fascicles.
The manner in which the tumor infiltrates the subcutaneous tissue is an extremely important histologic feature of DFSP and is frequently of diagnostic value. While the honeycomb pattern is the best-known pattern in DFSP, the parallel-band pattern has been found to be more common in certain series.9 In our case, the predominant pattern was the honeycomb pattern (46.6%), followed by the digitiform pattern (27.1%), the compressive pattern (14.5%), and the parallel-band pattern (11.6%). The honeycomb pattern, which is an important diagnostic clue, was actually observed in the majority of tumors, even though it was not the predominant pattern. Nonetheless, it should be noted that identification of subcutaneous infiltration patterns is prone to subjective interpretation and it is therefore difficult to establish the frequency of one pattern or another with certainty. The parallel-band pattern has been associated with a shorter disease course in DFSP, while the honeycomb pattern has been associated with advanced disease and is actually considered to be representative of the final stage of tumor growth. We did not specifically study the association between disease course and subcutaneous patterns in our series, but as noted in the section below on factors associated with local aggressiveness, we did not find a significant association between subcutaneous infiltration patterns and a greater frequency of fascia involvement.
Fibrosarcomatous areas may be observed in between 7% and 20.9% of DFPS cases.14–16 The prevalence in our series—13.6%—is consistent with these figures. The prognostic implications of fibrosarcomatous areas in DFSP in relation to our findings and reports in the literature are discussed below. Other histologic variants or areas of degeneration are rare in DFSP, as reflected by reports in the literature and our findings. It is difficult to establish the frequency of these histologic subtypes; the most common ones in our series were sclerotic areas (15.5%), myxoid areas (9.7%, and pigmented areas (6.7%). Giant cell fibroblastoma was particularly rare in our series, and was observed in just 1 adult. In the largest series of giant cell fibroblastoma to date, 11.6% of cases were observed in adults over 40 years old, which is consistent with our finding.
The number of mitotic fields tends to be low in DFSP.1 In our series, the mean number of figures observed in 10 fields of view at ×40 magnification was 1.8, which is also consistent with figures in the literature. The largest number of figures was found in tumors with a fibrosarcomatous component, which, as we will discuss later, might have a role in the locally aggressive nature of certain tumors.
Regarding histologic depth of invasion, DFSP tumors infiltrate the fascia or muscle in 0% to 24.6% of cases, depending on the series.17–19 Of the 155 cases analyzed in our series, the tumor had invaded the subcutaneous tissue in 63.8% of cases, the muscle fascia in 7.1% of cases, the muscle in 22.5% of cases, and the periosteum in 3.2% of cases. The rate of deep invasion observed (29.6%) is slightly higher than that generally reported. Although DFSP is associated with a favorable prognosis, it should be recalled that most cases involving muscle invasion constitute a locally aggressive sarcoma, which has implications for surgical treatment and postsurgical complications and sequelae. Because this form of deep invasion tends to be focal rather than diffuse, the treatment of choice is MMS, as it enables a complete study of the deep margin and spares healthy tissue; this sparing of healthy muscle tissue has important implications in terms of surgical complications and sequelae.
In our series, 3 histologic features were significantly associated with invasion of the muscle fascia or the galea aponeurotica: histologic pattern, cellular pleomorphism, and number of mitotic figures.
In the first case, both the sheetlike and cartwheel patterns were significantly associated with infiltration of the fascia or galea aponeurotica, indicating that particular attention should be paid to tumors with a predominance of either of these patterns. The general lack of cases with a predominant sheetlike pattern, however, probably indicates that our results were a chance finding. The most common histologic pattern observed in DFSP is the storiform pattern, and in our series, this pattern was present in an almost identical proportion of tumors with deep (fascial) and superficial involvement.
In the second case, a high degree of cellular pleomorphism was significantly associated with deep invasion. DFSP is by definition a tumor with minimal pleomorphism, and when present, pleomorphic cells tend to be found largely in fibrosarcomatous areas, which have been identified as an adverse prognostic factor.17 Degree of cellular pleomorphism could thus be considered to be a relevant factor when it comes to predicting deep invasion in DFPS. Marked pleomorphism has also been identified as an important prognostic factor for recurrence and metastasis.14,17,20,21
Finally, our finding that the presence of more than 1 mitotic figure was significantly associated with a greater risk of invasion of the fascia or galea aponeurotica is consistent with reports in the literature, which have shown high mitotic activity to be significantly correlated with recurrence17 and to be linked to a 14% increased risk of death.20 Number of mitotic figures has also been associated with the presence of fibrosarcomatous areas.14
The retrospective design of this study constitutes a limitation, which could be overcome by performing a long-term prospective study to demonstrate that the factors we found to be significantly associated with deep invasion are more common in cases of recurrence. Such a study is challenging, however, considering that MMS, the treatment of choice for DFSP, is associated with low recurrence rates.
In conclusion, our results show that greater attention should be paid to cases of DFSP with a sheetlike and cartwheel pattern, a high degree of cellular pleomorphism, and more than 1 mitotic figure, as these factors appear to be associated with a greater risk of involvement of the muscle fascia. Finally, the fact that almost 30% of the tumors in our series had invaded the fascia or muscle should be taken into account when planning surgical treatment.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Serra-Guillén C, Llombart B, Nagore E, Guillén C, Requena C, Kindem S, et al. Estudio de los factores histológicos asociados a la infiltración en profundidad en el dermatofibrosarcoma protuberans. Actas Dermosifiliogr. 2016;107:414–420.
This study was awarded the Antonio Llombart Rodriguez-FINCIVO prize for 2015.