The interferons are a group of more than 20 glycoproteins produced by numerous body cells in response to the presence of viruses, double-stranded RNA, polypeptides, or bacterial products. All of them share the ability to inhibit viral replication and cell proliferation, and to regulate and modulate immune cells.1 From an immunologic point of view, interferons increase the expression of class I or II molecules of the major histocompatibility complex and stimulate natural killer (NK) lymphocytes.
There are 2 types of interferon: type 1, which is divided into alpha, beta, omega, and tao, and type 2 which comprises gamma interferon. The principal interferons of medical interest are alpha interferons, produced mainly by leukocytes, beta interferons, produced by fibroblasts, and gamma interferons, produced by T lymphocytes and NK cells. Interferons are primarily used in medicine to treat malignant neoplasms, viral diseases, and autoimmune diseases. Type 1 is more effective as an antiviral agent and type 2 has a more specific effect on regulation of the immune response.1
We describe a 22-year-old woman with an 8-month history of multiple sclerosis. She had been receiving interferon beta-1a subcutaneously at doses of 22mcg 3 times weekly for the previous 3 months. The patient reported depigmentation around nevi on her skin, with onset of the depigmentation only a few weeks after interferon therapy was started. She had no personal or family history of vitiligo and presented no previous halo nevi.
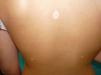
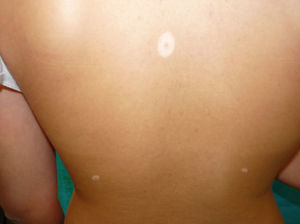
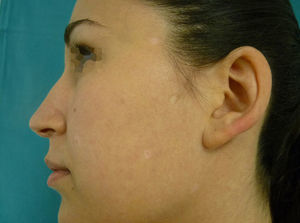
Physical examination revealed an achromic hypopigmented halo around almost all the nevi on the patient's skin, a total of 15, most of them on the back (Fig. 1) as well as around several intradermal nevi on the face (Fig. 2). The nevi were stable and had been present for some time; none presented clinical or dermoscopic atypia.
The interferon was well tolerated, with no liver or thyroid abnormalities and with satisfactory control of multiple sclerosis, which was in remission at the time.
Halo nevi express an autoimmunity phenomenon that manifests as an achromic hypopigmented halo around the nevus, which often disappears. Histology shows a lymphohistiocytic infiltrate directed against the melanocytes. Immune involvement in the genesis of this phenomenon is supported by the presence or increased numbers of T lymphocytes, mainly CD8+ cells, and antigen-presenting cells at the site of depigmentation.2,3 Additionally, the local endogenous production and activation of type 1 interferon have been seen to be involved in the regression of melanomas and other melanocytic lesions.3
Interferon beta-1b and 1a are approved by the Food and Drug Administration for the treatment of relapsing-remitting multiple sclerosis. They have proven to be disease-modifying drugs that can prevent exacerbations or lengthen the time between them.4 The drugs acts as immunomodulators, activating cytotoxic CD8+ T cells, and may modify the immune response. The most common side effects are inflammatory reactions at the injection site and flu-like symptoms. Asymptomatic elevation of liver enzymes and cytopenias are also observed. Rarely, they can cause various autoimmune phenomena such as thyroiditis, myasthenia gravis, rheumatoid arthritis, lupus erythematosus, and Raynaud phenomenon.4.5 The appearance of autoimmune hepatitis has also been described in a few reports.5 Autoantibodies, such as thyroglobulin and microsomal autoantibodies, have been detected occasionally with administration of the drug, but their significance is uncertain.6
Our report is the first to relate the appearance of multiple halo nevi with the use of interferon beta-1a. Another report describes a patient on treatment with interferon beta-1a who developed vitiligo that improved when the drug was discontinued.7 The authors postulated that interferon beta-1a could stimulate CD8+ lymphocytes to recognize melanocyte-derived proteins; these cells are known to play a key role in vitiligo. Several cases of vitiligo with the use of interferon alpha-2a have also been described.8
Although the exact pathophysiology of halo nevi and vitiligo is unknown, the 2 manifestations appear to have a common pathophysiology and often present together. Several theories suggest that these manifestations are the result of an immune response against melanocytes, probably mediated by CD8+ cell activation, as might occur with the use of interferon.
A literature review found 2 reports of drug-induced multiple halo nevi of sudden onset with the use of infliximab and imatinib.9,10 In the first case, the condition was associated with worsening of previous alopecia areata, and the authors proposed that anti-TNF drugs induce autoimmune phenomena that include alopecia areata and halo nevi. In the second case, there was a relationship with c-Kit tyrosine kinase inhibition.
In conclusion, our patient developed multiple halo nevi a few weeks after the start of interferon therapy. We believe that in our patient there was a relationship between the use of interferon and the development of multiple halo nevi, due to the temporal association and the immune system modifications that this therapy induces through CD8+ cell activation, favoring autoimmune phenomena; this is an important event in the pathophysiology of the development of halo nevi. The use of systemic or intralesional interferon has been shown to shrink or eliminate some melanoma metastases, probably by the same mechanism that induces the formation of halo nevi and vitiligo. This highlights the importance of immunity in the biology of melanocytic lesions and opens the door to possible therapeutic approaches to melanoma treatment.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vera-Iglesias E, et al. Halo nevi asociados al tratamiento con interferón beta-1a. Actas Dermosifiliogr. 2012;103:75–6.