Acute graft-versus-host disease (GvHD) is a life-threatening complication following allogeneic hematopoietic stem cell, or solid organ transplantation.1 Discriminating grade IV cutaneous GvHD from Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is challenging due to the similar clinical and histopathological features shared by both entities.2 However, distinguishing between the two diagnoses is important because the management of these conditions is not the same.1,3
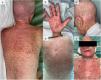
We report the case of a 61-year-old woman with myelodysplastic syndrome with excess blasts type 2 who underwent allogeneic stem cell transplantation. One day prior to the transplant, GvHD prophylaxis was initiated with cyclosporine A. The patient received multiple drugs at admission, including antibiotics, such as beta-lactams, trimethoprim/sulfamethoxazole, and vancomycin. One month later, an extensive maculopapular rash with skin detachment and a positive Nikolsky's sign appeared on the trunk, scalp (including the retroauricular region), palms, and soles (Fig. 1). Additionally, oral, genital, and ocular mucous membranes were affected as well. There were no signs of GI or hepatic involvement. Clinical diagnosis of GvDH vs SJS/TEN was considered. The skin biopsy confirmed the presence of a completely necrotic epidermis detached from the dermis, which was consistent with both diagnoses. The patient was, therefore, admitted to the ICU, suspected drugs were withdrawn, and treatment with prednisone (1mg/kg/12h) and immunoglobulin (1mg/kg/day) was initiated. However, skin lesions fail to improve after 48h.
Currently, there are no established biomarkers that can reliably diagnose GvHD. According to a pilot study, a composite biomarker panel including elafin, regenerating islet-derived 3-α, and soluble interleukin-2 receptor-α can be useful to differentiate acute GvHD from non-GvHD patients at onset with specificity and sensitivity rates of 100% and 55.6%, respectively.4 Nevertheless, these biomarkers are not widely available, which is why this analysis could not be conducted.
Flow cytometric analysis of skin blister fluid cells (BFC) was performed, and the results became available in <1h. The CD8+/CD4+ ratio among the CD3+ T cells found in our analysis was 2.1, and natural killer (NK) cells (CD3-CD56+ lymphocytes) accounted for up to 65.5% of the entire lymphocyte population (Fig. 2). In our centre, this evaluation is always conducted in available BFC of SJS/TEN cases following the protocols established by the PIELenRed consortium, a Spanish interdisciplinary platform for the investigation of severe cutaneous adverse drug reactions.5 The mean CD8+/CD4+ ratio in 10 SJS/TEN samples analyzed was 5.5 (range, 2.3–12.4), and the mean percentage of NK cells, 11.5% (range, 3–18%). As far as we know, no studies have ever compared the amount of CD8+, CD4+ and NK cells in blister fluids of GvHD and SJS/TEN. Nevertheless, according to Naik et al., the CD8+/CD4+ ratio in the histopathological analysis of grade IV GvHD skin biopsies was lower vs SJS/TEN (1.78 vs 7.33).6 Furthermore, Wegner et al. reported an increased number of NK cells in GvHD skin samples vs SJS/TEN.7 Therefore, immunohistochemical studies in skin biopsies and flow cytometric analyses of blister fluid may have parallel results. In fact, the findings of our case support the diagnosis of GvHD.
Dot plots representing the percentage of cell types found in the skin blister fluid by flow cytometric analysis. The LR square (CD3− CD56+) illustrates the NK cells. The first diagram shows our patient's data, with 65.5% of NK cells. The diagram below is an example of an SJS/TEN case, with only 7.6% of NK cells. GvHD, graft-versus-host disease; BFC, blister fluid cells; SJS/TEN, Stevens–Johnson syndrome/toxic epidermal necrolysis; NK cells, natural killer cells; UL, upper-left; UR, upper-right; LL, lower-left; LR, lower-right.
Finally, taking the patient's past medical history, clinical signs, and flow cytometric data into consideration, the diagnosis of GvHD was considered more likely, and targeted treatment was initiated with ruxolitinib and photopheresis, with rapid improvement of skin lesions. Unfortunately, the patient developed infectious and haemorrhagic complications leading her death on day +57.
One of the most important reasons for performing flow cytometric analysis of BFC instead of just a conventional biopsy is to help in the differential diagnosis of two entities whose histopathological findings often overlap, causing a diagnostic dilemma.2,6,7 In addition, we should emphasize that the results of this test can be obtained very quicky: in <1h in our centre. This can be crucial for decision-making and to start treatment fast.
Although the flow cytometric analysis of BFC is not a currently validated technique, results can likely be parallel to those found in immunohistochemical studies. In the future, this test may be a fast and useful tool to differentiate grade IV GvHD from SJS/TEN, which warrants further investigation.
FundingThis study was supported by a grant from Instituto de Salud Carlos III, Madrid, Spain (Spanish Ministry of Economy and Competitiveness)FIS PI18/00718 (co-funded by FEDER) to TB.
Conflict of interestThe authors declare that they have no conflict of interest.