The member of the phosphatidylinositol 3-kinase family, mammalian target of rapamycin, is involved in modulating inflammatory response and regulating cellular processes associated with growth, differentiation, and angiogenesis. Recent years have seen major advances in our understanding of the mammalian target of rapamycin signaling pathway and the implication of this pathway in multiple genetic and inflammatory diseases and tumors. The development of the mammalian target of rapamycin inhibitors has given rise to new treatment approaches that have led to substantially improved outcomes in many diseases. In this article, we review the role of the mammalian target of rapamycin signaling pathway in the different skin diseases with which it has been associated, examine the therapeutic applications of drugs targeting this pathway, and provide an overview of current trends and future directions in research.
La molécula diana de la rapamicina en mamíferos es una cinasa perteneciente a la familia de fosfatidil-3-inositol que está involucrada en la regulación de diferentes procesos relacionados con el crecimiento y diferenciación celular, la angiogénesis y la modulación de la respuesta inflamatoria. En los últimos años hemos presenciado un profundo avance en el conocimiento de las bases moleculares de la vía de señalización de la molécula diana de la rapamicina en mamíferos y su implicación en multitud de enfermedades genéticas, inflamatorias o tumorales. El desarrollo de moléculas inhibidoras de esta vía ha propiciado una nueva posibilidad de abordaje terapéutico que ha permitido una mejora sustancial en muchas de estas enfermedades. En este artículo revisamos las implicaciones de la vía de la molécula diana de la rapamicina en mamíferos en las diferentes dermatosis con las que se ha relacionado, sus aplicaciones farmacológicas y las futuras direcciones que están tomando las diferentes líneas de investigación.
The mammalian target of rapamycin (mTOR) is a serine/treonin kinase that regulates the phosphatidylinosital-3-kinase (PI3K)/Akt molecular signaling pathway. In 1965, a Canadian expedition to Easter Island (Rapa Nui) isolated a bacterium named Streptomyces hygroscopicus from soil samples. One of the fermentation products of this bacterium had antifungal properties. This product formed the basis for the development of rapamycin (also known as sirolimus). Subsequently, this molecule has shown potent immunosuppressive, antiproliferative, and antiangiogenic activity through inhibition of the mTOR pathway. Investigation into the pathways implicated in mTOR regulation has made considerable progress in recent decades. The pathophysiology associated with this pathway is, as a result, much better understood and new inhibitors of the mTOR pathway have been developed with different therapeutic indications.
The objective of this article is to review the impact of the mTOR pathway on the pathophysiology of different dermatoses with which it has been associated, and also to assess the safety and efficacy of inhibitors of this pathway that are currently available or in development.
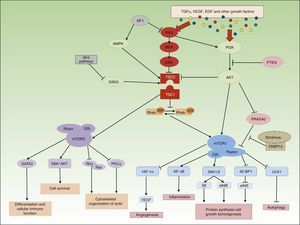
Molecular Basis of the mTOR PathwayThe mTOR pathway is a basic molecular signaling pathway in the regulation of cell metabolism, proliferation, and differentiation and it also plays an important role in immune system modulation and autophagy mechanisms.1 mTOR is a protein included in the PI3K-kinase family. It comprises 5 protein domains with different functions, with binding to other proteins to form complexes with particularly noteworthy kinase activity. In 2002, Kim et al.2 found that mTOR formed 2 multiprotein complexes well-differentiated from each other both in their composition and molecular signaling functions. These complexes are known as molecular target of rapamycin complex 1 (mTORC1) and mTORC2. mTOR responds to different types of signal, such as growth factors, cytokines, mitogens, insulin, amino acids, and cell stress. These mediators activate the PI3K/Akt pathway, leading to phosphorylation of the protein tuberin (TSC2), which is bound to hamartin (TSC1) forming the TSC1/TSC2 complex. Phosphorylation leads to rupture of the hamartin-tuberin complex, which no longer inhibits conversion of Rheb-GDP to Rheb-GTP and mTORC1 is activated. The MAPK/ERK pathway also activates mTORC1 through dissociation of the TSC1/TSC2 complex. Other factors that downregulate this pathway are AMPK, PRAS40, HIF-1, and the Wnt pathway through GSK3 (Fig. 1).
Activation of mTORC1 has different consequences in cell homeostasis. The main one is its implication in regulation of cell growth and regulation. mTORC1 promotes protein synthesis, ribosomal biogenesis, mRNA transcription, and synthesis of lipids and nucleotides through different ribosomal mediators such as S6K and 4E-BP1.3 mTORC1 also intervenes in the regulation of autophagy, the process of self-degradation of cell components with the objective of maintaining homeostasis in normal or stress conditions. mTORC1 inhibits Ulk1, leading to activation of autophagy mechanisms to obtain energy and eliminate damaged organules.4 Prolonged inhibition of these autophagy mechanisms has been associated with the development of neurodegenerative diseases, epilepsy, and autism due to intracellular accumulation of protein aggregates. The mTOR pathway plays a fundamental role in the regulation of both innate and acquired immunity. mTORC1 and mTORC2 control the differentiation, maturation, and function of antigen-presenting cells and of B and T cells through NF-kB transcription factor and GATA3, respectively, although the specific mechanism of action by which the 2 complexes modulate the immune system is not known.5 The angiogenic activity of mTOR is essentially exercised through translation and activation of HIF-1, a molecule that is associated with VEGF expression in situations of cell hypoxia and that maintains mTORC1 innactive.6 Although less is known of the molecular functions of mTORC2, this complex has been associated with regulation of cell survival and organization of the actin cytoskeleton through the Rho/rac pathway.
Hyperactivation of this mTOR pathway has been associated with different genetic disorders and neurodegenerative, metabolic, and immune-mediated diseases. In addition, this pathway has been implicated in uncontrolled proliferation processes, malignant transformation, and chemoresistance in different types of cancer.
mTOR InhibitorsThe knowledge and investigation of the mTOR pathway has led to the design of pharmacological agents that target this molecular signaling cascade. Sirolimus was the first in the class of mTOR inhibitors. It was initially marketed as an immunosuppressive agent in transplant patients. Subsequently, other derivative drugs have been developed, such as everolimus and temsirolimus, and new indications have been added in different types of advanced cancer due to their antiproliferative action (Table 1).
mTOR Inhibitors Currently Approved by the European Medicines Agency.
| Drug | EMA Approval | Indications | Route of Administration | Very Common Adverse Effects (>10%) | Cutaneous Adverse Effects |
|---|---|---|---|---|---|
| Sirolimus | Rapamune (2001) | Prophylaxis against renal transplant rejection | Oral | Edema, headache, fever, arterial hypertension, GI disorders, anemia, thrombocytopenia, hyperlipidemia, arthralgia, diabetes mellitus, infections | (>10%): PPP; (<10%): delayed wound healing, stomatitis, exanthema, skin cancera; (<0.1%): lymphedema |
| Everolimus | Afinitor (2009) | Advanced breast cancer, advanced RCC, neuroendocrine tumors of pancreatic origin | Oral | Infections, anemia, anorexia, hyperglycemia, hyperlipidemia, headache, pneumonitis, epistaxis, GI disorders | (>10%): exanthema, pruritus, stomatitis; (<10%): PPP, xerosis, nail disorder, PPED; (<0.1%): angioedema |
| Certican (2003) | Prophylaxis against kidney, heart, and liver transplant rejection | Oral | Respiratory infections, UTI, leukopenia, thrombocytopenia, anemia, hyperlipidemia, diabetes, insomnia, headache, pericardial and pleural effusion, hypertension, dyspnea, GI disorder, edema | (<10%): PPP, exanthema, stomatitis, skin cancer, angioedema, delayed wound healing | |
| Votubia (2011) | Renal angiolipomas associated with TSC, SEGA associated with TSC | Oral | URT infections, hyperlipidemia, amenorrhea | (>10%): PPP, stomatitis; (<10%): exanthema, xerosis, pruritus, alopecia; (<0.1%): angioedema | |
| Temsirolimus | Torisel (2007) | Advanced RCC, MCL (second line) | Intravenous | Anemia, thrombocytopenia, leukopenia, GI disorder, edema, astenia, epistaxis, hyperglycemia, hyperlipidemia, infections, pneumonia, dyspnea | (>10%): exanthema, pruritus, xerosis, stomatitis; (<10%): dermatitis, nail disorders, PPP, purpura |
| Ridaforolimus | In development | Advanced endometrial carcinoma, metastatic sarcomas | Oral, intravenous | Infections, fatigue, thrombocytopenia, GI disorder, anemia, hyperlipidemia, headache, anorexia, dyspnea, neutropenia | (>10%): stomatitis, exanthema |
Abbreviations: EMA, European Medicines Agency; GI, gastrointestinal; MCL, mantel cell lymphoma; PPED, palmoplantar erythrodysesthesia; PPP, palmoplantar pustulosis; RCC, renal cell carcinoma; SEGA, subependymal giant cell astrocytoma; TSC, tuberous sclerosis complex; URT, upper respiratory tract; UTI, urinary tract infections.
The association of the use of sirolimus with the onset of skin cancer mentioned in the product labelling may be due to a bias in the pivotal trials to support approval. In these, the drug was combined with cyclosporin and corticosteroids in comparison with placebo to assess efficacy and safety in the prevention of graft rejection. This onset of carcinomas in certain subjects in the exposed group may be mainly due to the carcinogenic action of cyclosporin, as shown by subsequent studies in which the use of sirolimus monotherapy has not only been shown not to induce the development of skin cancers but also has a protective effect against their development.58
Source: Data obtained from the Summary of Product Characteristics available from http://www.ema.europa.eu
The mechanism of action of mTOR inhibitors is based on inhibition of the mTOR kinase activity through binding to the immunophilin FKBP12. This inhibition exerted by the sirolimus/FKBP12 complex blocks activation of protein synthesis and detains the process in the G1 phase of the cell cycle. The result is antitumoral action (thanks to activation of cellular apoptosis, reduction in VEGF expression, and inhibition of migration and cell invasion) and an immunodulatory action (as a result of suppression of activation and proliferation of T cells and decreased antibody production).
Metabolism of sirolimus and other mTOR inhibitors is mediated by the 3A4 cytochrome. This pharmacokinetic conditioning factor should be taken into account given that many drugs used in everyday dermatology practice may interact with mTOR inhibitors either inhibiting their activity (antifungals, macrolides, cyclosporin, imatinib) or enhancing it (rifamycin, dexamethasone).
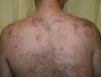
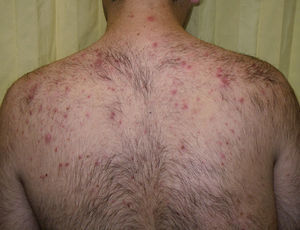
A wide range of adverse effects have been reported and these vary with mTOR inhibitor used, the dose, and the underlying diseases. In general, there is a greater predisposition to infections and gastrointestinal, hematological, and metabolic disorders (Table 1). Skin toxicity is often observed.7 The most common dermatological disorders are stomatitis, exanthemas (Fig. 2), and nail disorders (Table 2).
Papulopustular eruption in a patient in treatment with everolimus for subependymal giant cell astrocytoma associated with tuberous sclerosis. Clinically, this eruption resembles the eruption that develops with epidermal growth factor inhibitors. It is characterized by the appearance, 2 to 4 weeks after starting treatment, of monomorphic papules and follicular center pustules, with variable pruritus, without comedones or cysts, with a predilection for the trunk.
Adverse Cutaneous Effects of mTOR Inhibitors.
| Skin Toxicity (Frequency) | Clinical Characteristics | |
|---|---|---|
| Frequent (>10%) | Stomatitis (25%-44%) | Superficial sores Differential diagnosis: chemotherapy-induced stomatitis (ulcers with pseudomembranes) |
| Exanthemas (27%) | Morbilliform Eczematous Papulopustular | |
| Nail disorders (5%-46%) | Paronychia (+frequent) Onycholysis Nail fragility Yellow coloration Pyogenic-like granuloma lesions Dystrophy of all 20 nails | |
| Uncommon (<10%) | Delayed wound healing Edema Pruritus Xerosis Alopecia Hypertrichosis Leukocytoclastic vasculitis |
The antiproliferative, immunomodulatory, and antiangiogenic properties of mTOR inhibitors have led to their off-label use in a range of dermatological diseases of genetic, inflammatory, or tumoral origin (Table 3).
Studies of the Use of mTOR Inhibitors in Genetic Disorders Associated with Skin Manifestations.
| Genodermatoses | Molecular Targets | Most Frequent Dermatological Manifestations | mTOR Inhitor Use | Studies (Year) |
|---|---|---|---|---|
| Tuberous Sclerosis Complex | TSC1, TSC2 | Hypomelanotic macules Facial angiofibromas Subungual fibromas Connective tissue nevus | Oral and topical everolimus, oral and topical sirolimus | Wataya-Kaneda (2011, 2012, 201519), Salido et al.16 (2012), Foster et al.14 (2012), Koenig et al.15 (2012), etc. |
| RASopathies | ||||
| Neurofibromatosis 1 | NF1 | CaLM, axillary and inguinal freckling, neurofibromas, xanthogranulomas | Oral and topical sirolimus, oral everolimus | Weiss et al.23 (2015), Koenig (in progress) |
| Hamartomatous Polyposis Syndromes | ||||
| Peutz-Jeghers syndrome | STK11, LKB1 | Mucocutaneous lentigines | Oral everolimus | Klumpen et al.28 (2011) |
| Juvenile polyposis syndrome | SMAD4, BMPR1A | Hereditary hemorrhagic telangiectasis (SMAD4) | Oral sirolimus | Skaro et al.29 (2006) |
| PHTS | ||||
| Cowden syndrome Lhermitte-Duclos syndrome | PTEN | Trichilemmomas, fibromas, acral keratosis, hemangiomas, CaLM, lipomas | Oral sirolimus | Rajan (in development) |
| Bannayan-Riley-Ruvalcaba syndrome | Lipomatosis Pigmented macules of the glans penis | |||
| Proteus syndrome Proteus-like syndrome | Connective tissue nevus, lipomatosis, vascular malformations, epidermal nevi | Oral sirolimus | Marsh (2008) | |
| Muir-Torre Syndrome | MSH2, MLH1 (not related) | Sebaceous tumors, keratoacanthomas, BCC sebaceous differentiation | Oral sirolimus | Levi (2007), Griffard (2011) |
| Congenital Pachyonichia | K6a, K6b, K6c, K16, K17 | Palmoplantar keratoderma, subungual hyperkeratosis, epidermal cysts, oral leukokeratosis | Topical sirolimus | Teng (in progress) |
| Birt-Hogg-Dubé Syndrome | FLCN | Trichofolliculoma, trichodiscomas, fibromas | Topical sirolimus | Gijezen et al.24 (2014) |
| Familial Multiple Discoid Fibromas | Not described | Discoid fibromas | Topical sirolimus | Wee et al.25 (2013) |
Abbreviations: BCC, basal cell carcinoma; CaLM, café-au-lait macules.
Tuberous sclerosis complex (TSC) is a genodermatosis of autosomal dominant inheritance characterized by the multisystemic formation of hamartomatous tumors. The disease is caused by mutations in the TSC1 and TSC2 genes, leading to constitutive activation of mTORC1. This stimulation translates into uncontrolled cell proliferation and differentiation, resulting in the appearance of hamartomas in different tissues (such as skin, brain, and kidneys) and that are responsible for the clinical characteristics of these patients. The direct implication of the mTOR pathway in the pathogenesis of this disease has prompted the use of different mTOR inhibitors to palliate the skin and systemic manifestations. The good outcomes with oral everolimus in the treatment of subependymal giant cell astrocytomas8 and renal angiomyolipomas9 have led to these indications being added the labeling of this drug. Recent studies have also shown these drugs to be effective, with a good safety profile, in the treatment of lymphangioleiomyomatosis,10 leading to approval by the Food and Drug Administration of this indication in the case of sirolimus.11
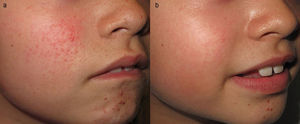
Among the multiple skin manifestations of TSC, hypomelanotic macules and facial angiofibromas (FAs) are the most frequent. Traditionally, treatment of FA was based on local destructive techniques such as CO2 laser or electrocoagulation, but these are associated with a high risk of sequelae.12 The substantial improvement observed in FA in a patient in treatment with oral sirolimus13 led to the use of different topical formulations for treatment with concentrations ranging from 0.015% to 1%.14–17 Good outcomes were attained with this regimen (Fig. 3), while minimizing potential side effects associated with systemic sirolimus administration. Local irritation was only observed in patients in whom a solution was applied (4.8% of cases),18 with the other formulations being well tolerated. Sirolimus has also been used topically with good response in hypomelanotic macules19 and in a patient with subungual fibromas.20 The topical formulation of everolimus has only been used in an isolated patient with FA.21
Neurofibromatosis Type 1 and Other RASopathiesRASopathies are developmental abnormalities caused by germline mutations in the RAS/MAPK pathway. They are characterized by a broad spectrum of skin and systemic manifestations.22 Neurofibromatosis and Legius, Noonan, Leopard, and cardiofaciocutaneous syndromes are all considered RASopathies. The link between the RAS/MAPK pathway and the PI3K/Akt/TSC/mTOR pathway has aroused interest in mTOR inhibitors as possible therapeutic alternatives in these patients. In a recent phase ii clinical trial, the use of oral sirolimus showed a modest decrease in plexiform neurofibroma growth with acceptable toxicity.23 Currently, a phase ii clinical trial is ongoing to investigate administration of oral everolimus in patients with neurofibromatosis type 1, while another phase i trial of the use of topical sirolimus for skin manifestations of this RASopathy has been completed although the results are not yet available.
Birt-Hogg-Dubé SyndromeBirt-Hogg-Dubé syndrome is characterized by mutations in the folliculin gene, which is implicated in the mTOR pathway. These patients have facial trichofolliculomas, lesions very similar to facial angiofibromas in TSC. However, topical application of sirolimus was of no benefit in these patients.24
Familial Multiple Discoid FibromasFamilial multiple discoid fibroma, which was initially included in the spectrum of Birt-Hogg-Dubé syndrome, is a rare entity characterized by early onset in the facial region of fibrovascular tumors denoted discoid fibromas. An article has been published describing improvement in the lesions of 2 twins with this disorder after treatment with 1% topical sirolimus.25
Pachyonychia CongenitaPachyonychia congenita is a disorder of autosomal dominant inheritance characterized by the appearance of painful focal palmoplantar keratoderma, subungual hyperkeratosis, epidermoid cysts, and oral leukokeratosis. It can present in a range of clinical forms depending on the mutations present in the genes responsible for encoding keratins K6a, K6b, K6c, K16, and K17. Sirolimus has been demonstrated to inhibit keratinocyte proliferation by blocking keratin K6a expression. Clinically, sirolimus has been associated with an ostensible improvement in plantar keratoderma in 3 patients treated with oral sirolimus.26 These findings have prompted a clinical trial with topical sirolimus for the treatment of this plantar keratoderma.
Hamartomatous Polyposis SyndromesHamartomatous polyposis syndromes include a group of genetic disorders of autosomal dominant inheritance, characterized by the development of gastrointestinal hamartomatous polyps, as well as other extradigestive manifestations including different dermatological conditions.27 These syndromes of hamartomatous polyposis include PTEN-associated hamartoma tumor syndrome, which covers a heterogenous group of clinical disorders sharing a PTEN germline mutation that suppresses the PI3K-Akt axis. A phase ii clinical trial of the use of oral sirolimus in patients with PTEN-associated tumor hamartoma syndrome has been completed and we are awaiting the results.
Klumpen et al.28 treated a patient with Peutz-Jeghers syndrome who developed a pancreatic carcinoma with oral everolimus. They observed a partial improvement in the tumor and the colonic polyps disappeared but the authors did not report on the effect on the patient's lentigines.
In cases in which juvenile polyposis syndrome is associated with SMAD4 mutations, the hereditary hemorrhagic telangiectasia or Rendu-Osler-Weber syndrome may also be present. There is a report of 1 case in which sirolimus led to remission of both the skin lesions and the intestinal lesions.29
Vascular AbnormalitiesCapillary Vascular MalformationsCapillary vascular malformations are congenital malformations that may appear sporadically or associated with genetic syndromes such as Sturge-Weber syndrome. Related to GNAQ mutations, up to 70% of cases associated with Sturge-Weber syndrome present activation of the Akt pathway.30 Recently, the results of a phase ii clinical trial have been published in which patients with capillary vascular malformations associated with Sturge-Weber syndrome were treated with pulsed dye laser light and topical rapamycin. The areas to which the combined therapy was applied showed a greater response compared with those treated with laser light only or placebo.31
PHACE SyndromePosterior fossa malformations–hemangiomas–arterial anomalies–cardiac defects–eye abnormalities–sternal cleft and supraumbilical raphe syndrome (more commonly known as PHACE syndrome) is characterized by association of large facial hemangiomas and other underlying vascular disorders, such as posterior fossa brain malformations, cerebral artery abnormalities, and cardiac, aortic, and ocular disorders. The large deformation and functional problems arising from these hemangiomas, as well as safety issues observed with the use of oral propanol in some of these patients, have prompted a search for therapeutic alternatives. A case has been published of good cutaneous response with oral sirolimus; no complications were observed in the other vascular disorders.32
Kaposiform HemangioendotheliomaKaposiform hemangioendothelioma is an uncommon childhood vascular tumor that can infiltrate deep structures such as muscle or bone. It is associated with the Kassabach-Merrit phenomenon, and is life-threatening. Sirolimus33 and everolimus34 have led to a decrease in or stabilization of the size of the lesions, as well as control of the associated symptoms (thrombocytopenia, coagulopathy) in isolated cases of kaposiform hemangioendothelioma refractory to other treatments.
Other Vascular AbnormalitiesCase reports have been published of the use of sirolimus in other vascular abnormalities, such as lymphatic and venous malformations,33 benign lymphangioendotheliomas,35 hemangioendotheliomas associated with Mafucci syndrome,36 blue rubber bleb nevus syndrome,37 and complex vascular malformations.
Inflammatory DiseasesGraft Versus Host DiseaseGraft versus host disease (GVHD) is a complication that can present in up to 80% of allogeneic stem cell transplant procedures. In a recent metaanalysis, treatment with sirolimus was shown to be useful in preventing the development of acute GVHD, with no signs of a decrease in relative risk in the chronic form.38 Both sirolimus39 and everolimus40 have been shown to be effective in controlling the symptoms of chronic GVHD with low rates of graft dysfunction and thrombotic microangiopathy. In addition, sirolimus showed promising results in patients with acute corticosteroid-resistant GVHD.41 However, mTOR inhibitors remain second-line therapies in the management of acute and chronic GVHD, behind corticosteroids whether used alone or in combination with calcineurin inhibitors.42,43 In a study in 34 patients with chronic sclerodermatous GVHD,44 mTOR inhibitors achieved an overall response rate of 76%, and so the authors considered sirolimus and everolimus as a good therapeutic option in these particularly refractory forms of the disease.
These results in patients with chronic GVHD have prompted the use of mTOR inhibitors in sclerosing diseases. Su et al.45 conducted a pilot clinical trial in 18 patients with diffuse systemic sclerosis in whom sirolimus showed a similar efficacy to methotrexate.
PsoriasisActivation of the PI3K/Akt/mTOR pathway in psoriatic plaques and perilesional skin46 has led to speculation about the potential use of mTOR inhibitors in psoriasis. A clinical trial provided evidence that the combination of sirolimus at subtherapeutic doses of cyclosporin (1.25mg/Kg) was as effective as high-dose cyclosporin, but with a better renal safety profile. However, when used in monotherapy, these agents did not produce any improvement in psoriasis.47 Likewise, the use of topical sirolimus at much higher concentrations than those used in other indications (2.2%-8%) has not shown any clinically relevant effects.48 There are only 2 clinical cases of oral everolimus use in patients with psoriasis.49,50 In both cases, the agent was combined with other immunosuppressants (cyclosporin and tacrolimus) that the patients had been taking previously to avoid graft rejection.
Autoimmune Bullous DiseasesThe use of sirolimus for prevention of suprabasal acantholysis in an animal model51 would explain the possible beneficial effect of mTOR inhibitor use in pemphigus vulgaris. However, current evidence in blistering diseases is limited to 3 cases of oral pemphigus treated with topical sirolimus without any improvement,52 and a case of pemphigus vulgaris treated with sirolimus in combination with high doses of intravenous immunoglobulins during the maintenance phase.53 Everolimus has recently been linked to the induction of 2 cases of bullous pemphigoid,54 and so the possible beneficial role of mTOR inhibitors in autoimmune bullous diseases has been brought into question
Oral Erosive Lichen PlanusThere is only 1 clinical study in patients with oral erosive lichen planus. In that study, Soria et al.55 treated 7 women with topical sirolimus. They obtained a complete response in 4 patients and a partial response in 3, and the formulation was well tolerated.
Skin OncologyNonmelanoma Skin CancerAlthough there are isolated cases of patients with basal-cell carcinoma treated with everolimus,56 prevention of nonmelanoma skin cancer is one the main uses of mTOR inhibitors. Several clinical trials have shown a decrease in the incidence of nonmelanoma skin cancer in transplant patients in treatment with sirolimus in comparison with calcineurin inhibitors. In a metaanalysis of 5876 transplant patients in 21 clinical trials, Knoll et al.57 observed a risk reduction for developing nonmelanoma cancer of 56%, as well as an overall decrease in the risk of cancer at any site in patients in treatment with sirolimus. The study showed a greater risk reduction in patients treated with other immunosuppressive therapies who subsequently switched to sirolimus.
MelanomaThere are 3 phase ii clinical trials with metastatic melanoma in which everolimus was administered in combination with other biologic agents such as bevacizumab,58 or associated with monochemotherapy (temozolomide)59 or polychemotherapy (paclitaxel+carboplatin).60 Although the combination with bevacizumab showed a modest benefit, with a response rate of 12% and stable disease in 58% of the patients, the use of everolimus with cytostatic agents did not show any benefit at all.
The association of temsirolimus with sorafenib in patients with metastatic melanoma was not efficaceous,61 whereas in combination with bevacizumab better results were obtained in patients without the BRAF mutation.62
Kaposi SarcomaSirolimus has been shown to be extremely effective in the treatment of skin and visceral involvement in Kaposi sarcoma in transplanted patients,63 and this has led some authors to consider the agent as the first-choice immunosuppressant in this subgroup of patients and even to use it topically.64
The limited experience with the use of everolimus in patients with Kaposi sarcoma is, on the other hand, more controversial. Although isolated clinical trials have been reported in which complete resolution of skin and systemic lesions has been attained,65 a recent clinical trial in 11 patients with classic Kaposi sarcoma showed limited efficacy66 with the onset of new lesions during treatment.
Cutaneous T-Cell LymphomasIn vitro studies have shown excessive activation of the mTOR pathway in cells of patients with Sézary syndrome and cutaneous T cell lymphomas, suggesting that mTOR inhibitors may be a possible therapeutic alternative.
Witzig et al.67 recently published a phase ii clinical trial in which patients with T-cell lymphomas were treated with oral everolimus. Seven of these had stage iiB (6) and stage iii (1) mycosis fungoides. Three of these (43%) attained partial response. Currently, there is a phase ii clinical trial ongoing to investigate treatment of refractory cutaneous T-cell lymphomas with everolimus.
AntiageingThe report in 2009 in Nature of the increased life span observed in mice who received sirolimus68 opened up a new avenue of investigation in the therapeutic applications of mTOR inhibitors, namely, cell ageing. The mTOR pathway has been implicated in the regulation of physiological mechanisms of geroconversion (irreversible passage of stem cells from quiescent to senescent cell stages). This cell cycle arrest leads to an irreversible weakening of the tissue regeneration processes and thus to ageing of the organism. Selective blockade of this step would keep the stem cells in quiescent state, thereby extending their regenerative function.
New mTOR Inhibitors and Future Lines of InvestigationCurrently, there are several studies in progress investigating the use of mTOR inhibitors in different types of dermatological diseases (Table 4). In addition, new mTOR inhibitors are appearing, although most are in the initial phases of development. Ridaforolimus is the only one with phase iii clinical data available in metastatic sarcomas.69 A slight increase in progression-free survival was observed but there was no benefit in terms of overall survival.
Main Ongoing Clinical Trials With mTOR Inhibitors in Dermatological Diseases.
| NCT number | Drug | Administration | Indication | Clinical Phase | Year of Initiation | Status |
|---|---|---|---|---|---|---|
| NCT01031901 | Sirolimus | Topical | Cutaneous manifestations of neurofibromatosis type 1 and TSC | Phase I | 2012 | Completed |
| NCT01853423 | Sirolimus | Topical | Facial angiofibromas in TSC | Phase I | 2013 | Recruiting |
| NCT02057614 | Sirolimus | Topical | Plantar keratoderma in congenital pachyonychia | Phase I | 2014 | Recruiting |
| NCT02123966 | Sirolimus | Topical | Chronic oral GVHD | Phase II | 2014 | Recruiting |
| NCT02214706 | Sirolimus | Topical | Capillary vascular malformations (sirolimus+erbium laser +PDL) | Phase II | 2014 | Recruiting |
| NCT00450320 | Sirolimus | Oral | HIV-associated Kaposi sarcoma | Phase I | 2007 | Completed |
| NCT00779194 | Sirolimus | Oral | Systemic lupus erythematosus | Phase II | 2008 | Ongoing |
| NCT00971789 | Sirolimus | Oral | Cowen syndrome and other PTHS | Phase II | 2009 | Completed |
| NCT01313923 | Sirolimus | Oral | Pemphigus | Phase 0 | 2011 | Recruiting |
| NCT00975819 | Sirolimus | Oral | Complex vascular abnormalities | Phase II | 2009 | Ongoing |
| NCT01811667 | Sirolimus | Oral | Complex vascular malformations | Phase III | 2012 | Recruiting |
| NCT02042326 | Sirolimus | Oral | Arteriovenous malformations | Phase II | 2014 | Recruiting |
| NCT02110069 | Sirolimus | Oral | Kaposiform hemangioendothelioma | Phase II | 2014 | Recruiting |
| NCT02509468 | Sirolimus | Oral | Slow-flow vascular malformations | Phase II | 2014 | Recruiting |
| NCT01637090 | Everolimus | Oral | Cutaneous T cell lymphomas | Phase II | 2012 | Recruiting |
| NCT01960829 | Everolimus | Oral | Metastatic melanoma | Phase II | 2013 | Recruiting |
| NCT01997255 | Everolimus | Oral | Sturge-Weber syndrome | Phase II | 2013 | Recruiting |
| NCT02332902 | Everolimus | Oral | Skin manifestations of neurofibromatosis type 1 | Phase II | 2014 | Recruiting |
Abbreviations: HIV, human immunodeficiency virus; GVHD, graft-versus-host disease; PDL, pulsed dye laser; PTHS, PTEN-associated tumor hamartoma syndrome; TSC, tuberous sclerosis complex.
The modest benefit observed with mTOR inhibitors in different oncology clinical trials performed to date may be due to the presence of multiple mTORC1 feedback mechanisms, such as activation of reflex signals of the PI3K and RAS/MAPK pathways. To avoid these proposed escape mechanisms, combination therapies with PI3K inhibitors and other cytostatic agents are being tested,70 and dual PI3K/mTOR or mTORC1/mTORC2 inhibitors are being developed.
ConclusionsThe PI3K/Akt/TSC/mTOR pathway is key in multiple molecular processes and has been associated with numerous diseases of very different characteristics. Its impact in dermatology has increased markedly in recent years as the pathogenic relationships with different dermatoses become known. This has opened a new therapeutic pathway with mTOR inhibitors, especially in their topically administered forms. To realize the potential of these treatments that target the mTOR pathway, research will be required in different fields. Use of existing molecules should be optimized, while new pharmacological agents should be developed. These should be associated with inhibitors of other associated signaling pathways.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Professor José Carlos Moreno Giménez for critically reading this article.
Please cite this article as: Salido-Vallejo R, Garnacho-Saucedo G, Vélez A. Bases moleculares y aplicaciones farmacológicas de la vía de mTOR en dermatología. Actas Dermosifiliogr. 2016;107:379–390.