Sweet syndrome is the most representative entity of febrile neutrophilic dermatoses. It typically presents in patients with pirexya, neutrophilia, painful tender erytomatous papules, nodules and plaques often distributed asymmetrically. Frequent sites include the face, neck and upper extremities. Affected sites show a characteristical neutrophilic infiltrate in the upper dermis. Its etiology remains elucidated, but it seems that can be mediated by a hypersensitivity reaction in which cytokines, followed by infiltration of neutrophils, may be involved. Systemic corticosteroids are the first-line of treatment in most cases. We present a concise review of the pathogenesis, classification, diagnosis and treatment update of this entity.
El síndrome de Sweet es la entidad más representativa de las dermatosis neutrofílicas. Por lo general se presenta en pacientes con fiebre, neutrofilia, pápulas erytomatosas dolorosas, nódulos y placas. Los sitios frecuentemente afectados incluyen la cara, cuello y extremidades superiores los cuales característicamente presentan un infiltrado neutrofílico en la dermis superior. Su etiología no esta bien establecida, pero parece que puede estar mediada por una reacción de hipersensibilidad de las citocinas, seguido por un infiltrado de neutrófilos. Los corticosteroides sistémicos son la primera línea de tratamiento en la mayoría de los casos. Se presenta una revisión actual de la patogénesis, clasificación, diagnóstico y tratamiento de esta entidad.
Since it was first described by Dr. Robert Douglas Sweet, originally known as Gomm-Button disease (in reference to the first two patients), Sweet's syndrome, also referred to as febrile neutrophilic dermatosis, has been reported in hundreds of patients worldwide.1 Neutrophilic dermatoses are a heterogeneous group of inflammatory skin disorders that include Sweet's syndrome, pyoderma gangrenosum, and subcorneal pustular dermatosis,2 the former being the most represented and the focus of this review.
The purpose of this article is to make a review of Sweet syndrome, its clinical manifestations, definition, pathogenesis, diagnosis and management of this entity. It is essential for dermatologists to know the different aspects of Sweet's syndrome as well as its proper diagnosis, prevention and treatment.
DefinitionClinically, Sweet's syndrome presents in patients, all of which show characteristic neutrophilic infiltrate in the upper dermis.3
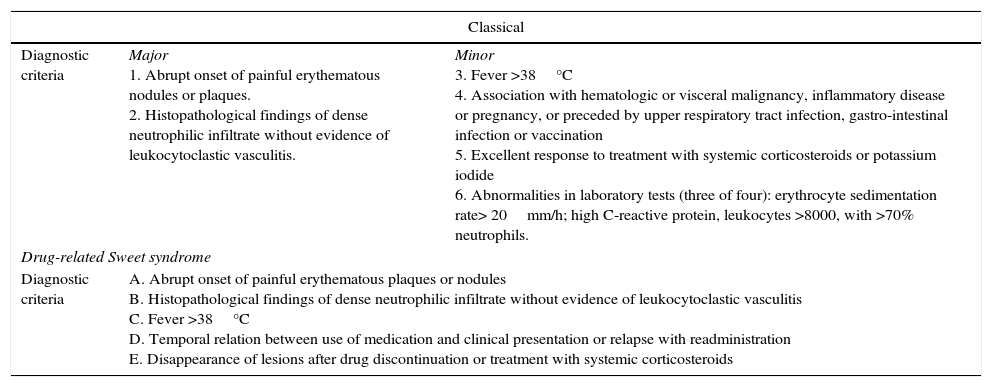
Sweet's syndrome can present as one of three clinical types: classical (or idiopathic) Sweet's syndrome, malignancy-associated Sweet's syndrome, or drug-induced Sweet's syndrome. Specific diagnostic criteria were proposed by Su and Liu4 and subsequently revised and modified by von den Driesch.5 Laboratory abnormalities may be found and are included in the diagnostic criteria, such as increased erythrocyte sedimentation rate (ESR), elevated C-reactive protein and leukocytosis (Table 1).
Diagnostic criteria for sweet syndrome, including classical, drug-induced and malignancy-associated forms.
| Classical | ||
|---|---|---|
| Diagnostic criteria | Major 1. Abrupt onset of painful erythematous nodules or plaques. 2. Histopathological findings of dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis. | Minor 3. Fever >38°C 4. Association with hematologic or visceral malignancy, inflammatory disease or pregnancy, or preceded by upper respiratory tract infection, gastro-intestinal infection or vaccination 5. Excellent response to treatment with systemic corticosteroids or potassium iodide 6. Abnormalities in laboratory tests (three of four): erythrocyte sedimentation rate> 20mm/h; high C-reactive protein, leukocytes >8000, with >70% neutrophils. |
| Drug-related Sweet syndrome | ||
| Diagnostic criteria | A. Abrupt onset of painful erythematous plaques or nodules B. Histopathological findings of dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis C. Fever >38°C D. Temporal relation between use of medication and clinical presentation or relapse with readministration E. Disappearance of lesions after drug discontinuation or treatment with systemic corticosteroids | |
In classical: The presence of both major criteria (1 and 2) and two minor criteria (3–6) is needed to establish the diagnosis of classical Sweet's syndrome. In malignancy-associated: the patients must precede, follow, or appear concurrent with the diagnosis of the patient's neoplasm and classical Sweet's syndrome diagnostic criteria. In drug-induced: All five features should be present to diagnose Sweet's syndrome. Adapted from Cohen et al.7
Sweet's syndrome is an inflammatory skin disorder characterized by the extensive infiltration of neutrophils into the epidermis and dermis. For a dermatologist, understanding the pathophysiology of Sweet's syndrome is crucial for treatment.
The underlining biological pathways responsible for this cutaneous neutrophilic dermatosis have remained elusive. However, the association of this disease with infection, autoimmune diseases, neoplasms and drugs suggests an unusual hypersensitivity that may be mediated by cytokines, followed by infiltration of neutrophils that are probably activated by interleukin (IL)-1. Circulating autoantibodies, cytokines, dermal dendrocytes, HLA serotypes, immune complexes and leukotactic mechanisms have been suggested as factors that contribute to the pathogenesis of this syndrome. The presence of IL-1, IL-2 and IFN-γ but not IL-4, suggests that type 1T helper cells may play a role in the pathogenesis of idiopathic varieties of this syndrome.6,7 Inflammatory cell markers, including CD3, CD163, myeloperoxidase, metalloproteinases and vascular endothelial growth factors, display significantly higher values in the lesioned skin of patients with Sweet's syndrome compared to non-Sweet's syndrome individuals or patients with other neutrophilic dermatoses.8
It has also been postulated that photosensitivity may play a role in the pathogenesis of Sweet's syndrome, although the pathomechanism is unknown, as Sweet's syndrome has been experimentally induced by phototesting.9 One theory suggests that an isomorphic Koebner reaction is at work; another proposed mechanism associates ultraviolet B radiation with neutrophil activation and epidermal production of tumor necrosis factor-alpha and interleukin-8.10
In malignancy-associated Sweet's syndrome, the most accepted theory of mechanism of pathogenesis is the overproduction and inappropriate regulation of inflammatory cytokines, such as IL-1, IL-3, IL-6, IL-8, granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF).11 This theory is supported by cases in which Sweet's syndrome patients received G-CSF/GM-CSF, interferon-γ and all-trans retinoic acid (ATRA) and subsequently developed Sweet's syndrome.11
Although there is no consistent evidence of a genetic predisposition for Sweet's syndrome, a higher frequency of HLA-Bw54 was reported in Japanese patients with Sweet's syndrome.6 However, analysis of the HLA antigens in a Caucasian population showed no association between this syndrome and specific HLA-ABC antigens.12 Recent evidence based on animal models suggest that an alteration in the gene encoding protein tyrosine phosphatase non-receptor type 6 (Ptpn6) could be involved in the pathogenesis of Sweet's syndrome,13 as it encodes non-receptor protein tyrosine phosphatase Src homology region 2 (SH2) domain-containing phosphatase-1 (SHP-1). The malfunction of Ptpn6 results in unremitting footpad swelling, suppurative inflammation, and neutrophilia. More studies are required to better understand the etiology of this disease.
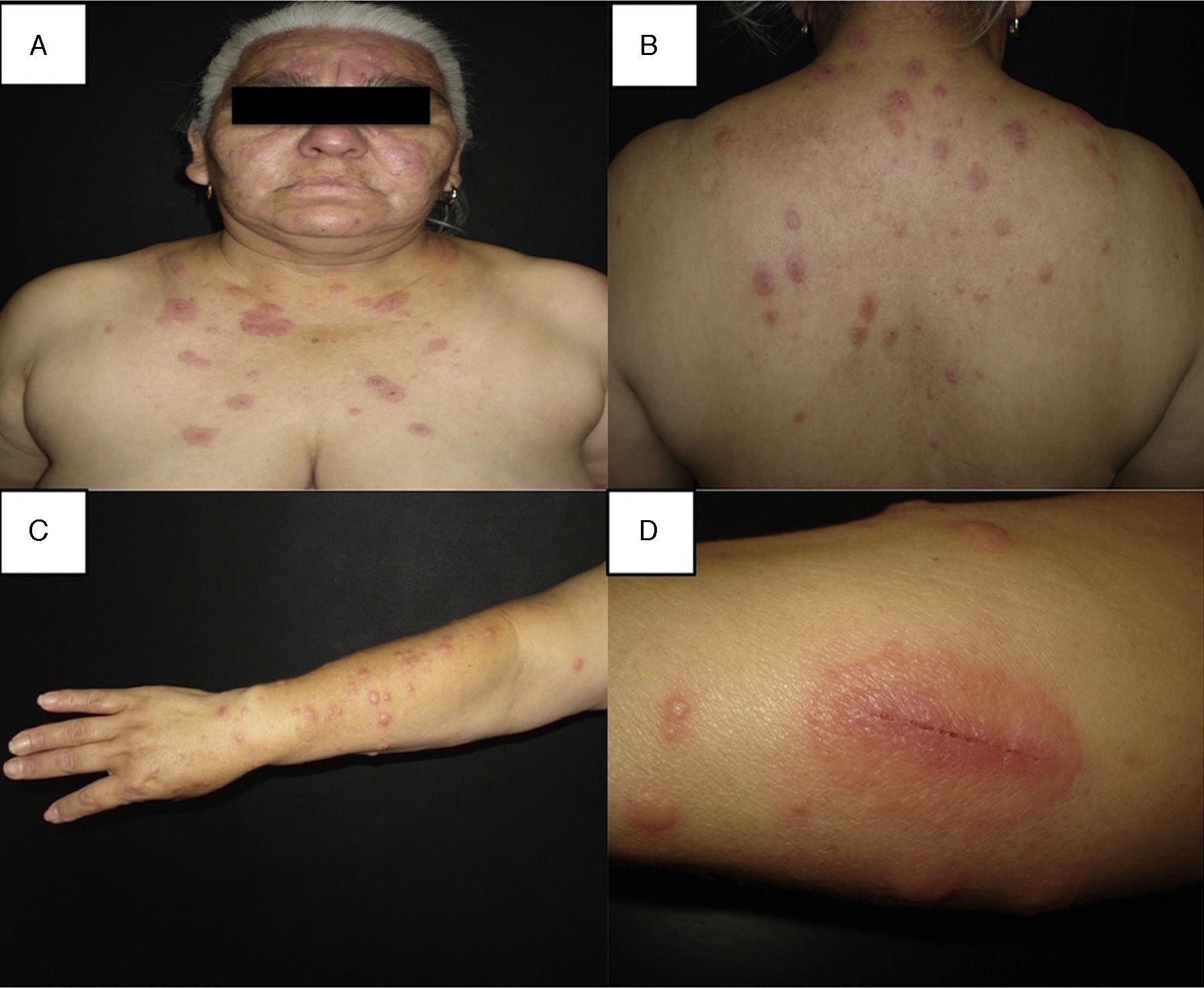
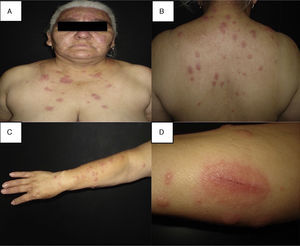
Clinical manifestationsClassical Sweet's syndrome has a worldwide distribution, usually presenting in middle age women with a 4:1 female to male ratio, no racial disparity, and recurrence in one-third of patients.3 It presents as an acute febrile neutrophilic dermatosis characterized by a constellation of clinical symptoms, physical features, and pathological findings that include fever, neutrophilia, asymmetrically distributed painful tender erythematous skin lesions, consisting of papules, nodules and plaques, usually affecting face, neck and upper extremities (Fig. 1). Histology reveals a characteristic diffuse infiltrate predominantly consisting of mature neutrophils typically located in the upper dermis, which tend to promptly improve after the initiation of treatment.7 Atypical lesions, characterized by erythematous plaques, vesicles and bullous lesions, have also been described.14 Typically, classical or idiopathic Sweet's syndrome may be associated with infection, usually of the upper respiratory (streptococci) or gastrointestinal tract (salmonellosis and yersiniosis), and inflammatory bowel disease.7 It can also occur during pregnancy,15 possibly related to the vascular, cellular, microbiological, and immunological changes linked to increased estrogen and progestogen levels during pregnancy.16 The symptoms and clinical manifestations typically respond promptly to systemic corticosteroid therapy and recurrence occurs in one-third of patients.7
(A–C) Disseminated dermatosis affecting the face, upper trunk and four limbs, predominantly on sun-exposed areas, bilateral and prone to symmetry, polymorphous, characterized by erythematous, thick, sharply demarcated nodules and plaques, and some yellow pustules between them. (D) Pathergy phenomenon.
Malignancy-associated Sweet's syndrome was first described by Cohen et al. 17. In this subtype, the clinical manifestations can precede, follow, or appear concurrent with the diagnosis of neoplasm in patients. Indeed, the dermatosis can be the cutaneous portent of either an undiagnosed visceral malignancy in a previously cancer-free individual or an unsuspected cancer recurrence in an oncology patient.11 Approximately 21% of Sweet's syndrome patients have an associated malignancy; 85% of these are linked to hematological disorders, most frequently to acute myelogenous leukemia (AML). Malignancy-associated Sweet's syndrome has also been described in patients with Hodgkin disease18 and polycythemia vera19 and in 15% of patients with solid tumors, principally adenocarcinomas of the breast, genitourinary tract and gastrointestinal tract.20 Extracutaneous manifestations are present in 50% of patients affected with malignancy-associated Sweet's syndrome21 and are more frequently caused by a hematological malignancy than a solid tumor. Therefore, it is important to maintain rigorous a follow-up in these types of patients, possibly contributing to the early detection and treatment of the underlining malignancy.
The syndrome may occur as a paraneoplastic accompaniment, appearing as a first sign of malignancy or its recurrence, usually meaning a poor prognosis,22 as it has been reported in cases of myelodysplastic syndrome (MDS).23 This last variant of Sweet's syndrome represents lot of controversies in the literature, as it has been associated with autoimmune disorders and abnormalities in chemokines which leads to a pro-inflammatory state,24 leading these patients to an increased instance of autoimmune skin disease including vitiligo, alopecia areata, eczema, vasculitis and pyoderma gangrenosum.25 It has also been suggested to consider MEFV gene analysis, the gene that is responsible for familial Mediterranean fever, in patients with MDS who have marked neutrophilia and antibiotic-resistant high fever or in those with Sweet's syndrome,26 although further evidence is required.
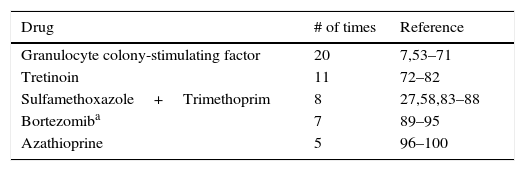
Finally, in drug-induced Sweet's syndrome, there is nearly always a temporal relationship between medication administration and symptom development. In 1996, Walker and Cohen described the diagnostic criteria (Table 1) for drug-induced Sweet's syndrome.27 The most commonly reported drug causing Sweet's syndrome is granulocyte-colony stimulating factor. Several anticancer agents, including all-trans-retinoic acid proteosome inhibitors, hypomethylating agents, tyrosine kinase inhibitors and lenalidomide are potential harbingers of Sweet's syndrome. Table 2 summarizes the top 5 reports of drug-induced Sweet's syndrome available in PubMed.
Sweet's syndrome can affect individually other organs, such as bones, brain, ears, eyes, kidneys, intestines, liver, heart, lung, mouth, muscles and spleen, particularly when associated with a malignancy that includes an extracutaneous site, which occurs in up to 50% of reported cases,7 or present in patients with multiorganic affection.28 Lungs are the most common extracutaneous site; symptoms range from upper respiratory tract infection with flu-like symptoms in its early stages to acute respiratory distress syndrome, and imaging demonstrates diffuse ground-glass opacities or consolidation. Radiological changes in the lungs include the presence of nodular, reticular or patchy infiltration, with or without effusion.29 Bronchoalveolar lavage is characterized by neutrophilic predominance with negative cultures.30 Some patients have been confirmed to have pulmonary involvement based on transbronchial lung biopsies, characterized by interstitial inflammation, edema and mild fibrosis, in which a large number of neutrophils and occasional lymphocytes, macrophages and eosinophils infiltrate the alveoli.31 Aortic stenosis, aortitis, cardiomegaly, coronary artery occlusion and myocardial infiltration with neutrophils have all been previously reported to occur in patients with heart involvement.21
Different varieties and atypical cases of Sweet's syndrome can occur. Neutrophilic dermatosis of the hands is currently considered to be a localized form that is capable of spreading to other locations and is not always accompanied by fever and neutrophilia.32 Another atypical localized form is neutrophilic dermatosis on the site of a lymphedema, which has a milder course with fewer systemic symptoms, fewer relapses, and typically responds well to oral antibiotics, anti-inflammatory drugs, and topical corticosteroids,33 although its etiogenesis remains undefined. Recently, Kroshinsky et al. described necrotizing Sweet's syndrome, a new variant of neutrophilic dermatoses characterized by the rapid onset of edematous, erythematous, warm cutaneous lesions with deep tissue neutrophilic infiltration and soft tissue necrosis in the absence of an infection, which tends to be cyclic with a high morbidity.34 Necrotizing Sweet's syndrome can occur as the first manifestation of HIV infection35 or dermatomyositis36 or in association with inflammatory bowel disease, Behçet's disease, relapsing polychondritis, rheumatoid arthritis or thyroid disease, including Graves’ disease and Hashimoto thyroiditis.37
In some patients with classical Sweet's syndrome, the symptoms and lesions of Sweet's syndrome are eventually resolved without any therapeutic intervention, while in others, symptoms and lesions can persist without treatment for weeks or months.7 Successful management of the cancer occasionally results after clearing the related dermatosis in patients with malignancy-associated Sweet's syndrome.17 Similarly, discontinuation of the associated medication in drug-induced Sweet's syndrome results in spontaneous improvement and subsequent resolution.27 Reports of fatal outcomes of Sweet's syndrome are uncommon, as it has been describe as an idiopathic chronic systemic inflammatory response syndrome.38 Recurrence is very common and remission between episodes is variable, occurring either after spontaneous remission or therapy-induced clinical resolution;2 therefore, it is crucial conduct follow-ups.
HistopathologyHistopathological analysis that characterizes a dense and diffuse dermal neutrophilic infiltrate is important for the diagnosis of the disease because the differential diagnosis of Sweet's syndrome is extensive.
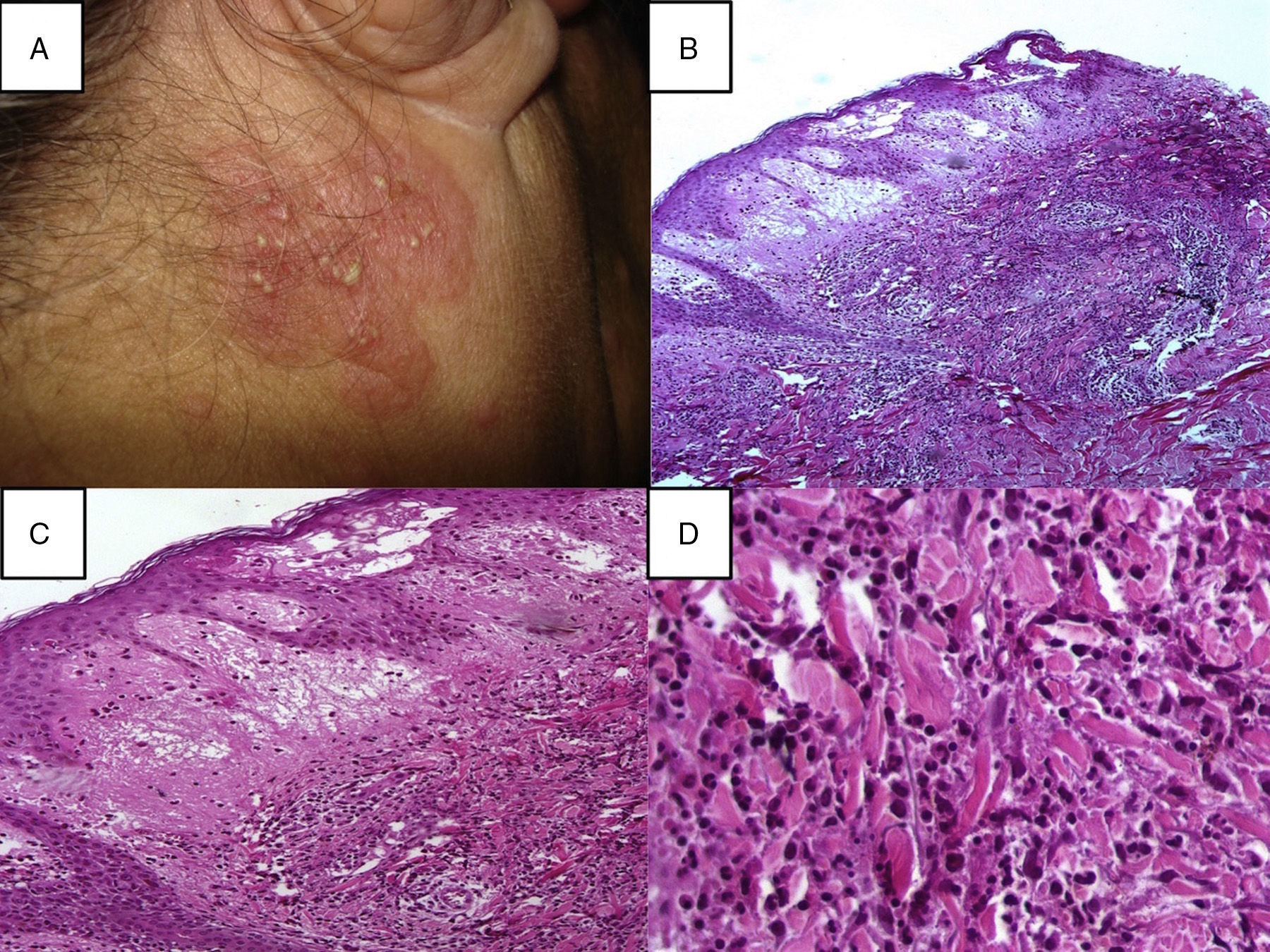
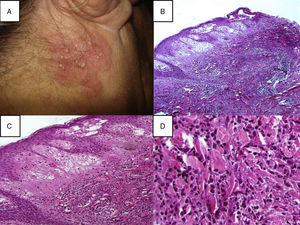
Histopathological diagnostic criteria include the presence of diffuse neutrophilic infiltrate in the dermis, edema, and fragmentation of the nuclei of neutrophils (Fig. 2). The predominant cells that comprise the infiltrate in the dermis of cutaneous Sweet's syndrome lesions are mature neutrophils; however, eosinophils have been observed within the dermal infiltrate in the skin lesions of some patients with either the classical or the drug-induced Sweet's syndrome. Occasionally, lymphocytes or histiocytes may also be present in the inflammatory infiltrate.9,39 The neutrophilic infiltrate may be perivascular, leading to leukocytoclastic vasculitis.7,40 The signs of this perivascular neutrophilic infiltration, which are not always seen on histopathological examination, are inflammatory infiltrate around postcapillary venules, with a predominance of neutrophils, nuclear dust, extravasation of erythrocytes, fibrin deposition in vessel walls, necrosis, and granuloma formation.41 This infiltrate is usually localized to the papillary and upper reticular dermis; however, neutrophils can also be present in the epidermis as either neutrophilic spongiotic vesicles42 or subcorneal pustules5; the infiltrate can also extend into the subcutaneous tissue and the hypodermis, affecting lobules of adipocytes and/or septa, recently reported as being associated with myeloid disorders.43 This subcutaneous infiltration, referred to as “subcutaneous Sweet's syndrome”, may lead to neutrophilic panniculitis in up to 38% of cases. However, the necrosis of adipocytes is clearly absent, even though a large number of neutrophils, and lymphocytes, monocytes, and multinucleated giant cells may also be found.44 The presence of subcutaneous neutrophilic inflammation in Sweet's syndrome lesions may be a more common finding in patients with either an associated hematologic dyscrasia or a solid tumor.
(A) A papulopustular lesion localizated on the head, in the posterior region of the right ear, a skin biopsy (Punch 4) was performed and stained with hematoxylin–eosin; (B) Histopathology reveals diffuse neutrophilic infiltrate with significant papillary dermal edema, 10×; (C) Papillary dermal edema, perivascular and interstitial infiltrate composed predominately of neutrophils, scattered lymphocytes, histiocytes, and eosinophils, 20×; (D) The infiltration was predominantly composed of neutrophils and histiocytes, 40×.
An unusual histopathologic variant that should be addressed is the histiocytoid Sweet syndrome. In this variant, fresh lesions are histopathologically characterized by an inflammatory infiltrate mostly composed of cells that may be misinterpreted as histiocytes, when in fact immunohistochemical studies demonstrate that they are immature neutrophils. These lesions probably result from the release of immature myeloid cells by the bone marrow in early acute stages of the disease, and these immature myeloid cells are replaced by mature neutrophils in later stages of evolution. Histopathologic differential diagnosis should be established with leukemia cutis and other inflammatory conditions characterized by histiocytes interstitially arranged between collagen bundles of the dermis. The lesions show a benign biological behavior and respond promptly to low doses of oral corticosteroids or nonsteroidal anti-inflammatory drugs.45
In a few rare cases of MDS, lymphocytic infiltrates are the presenting feature of Sweet's syndrome. Initially lymphocytic infiltrates in this subset could be attributed either to an early timing of the biopsy concerning the age of the lesion or to the dysgranulopoiesis syndrome. A possible relationship between the dysfunction of the receptor of the granulocyte-macrophage colony stimulating factor, the gene of which is located on the pseudoautosomal X-Y region, may exist in MDS patients with initially lymphocytic Sweet's syndrome. This could explain the male gender of this subset and might establish initially lymphocytic Sweet's syndrome as a distinguished clinicopathological entity for predicting the occurrence and even the prognosis of MDS.46
All of these characteristics of the infiltrate make a clinic-pathological correlation essential for confirming the diagnosis of Sweet's syndrome.
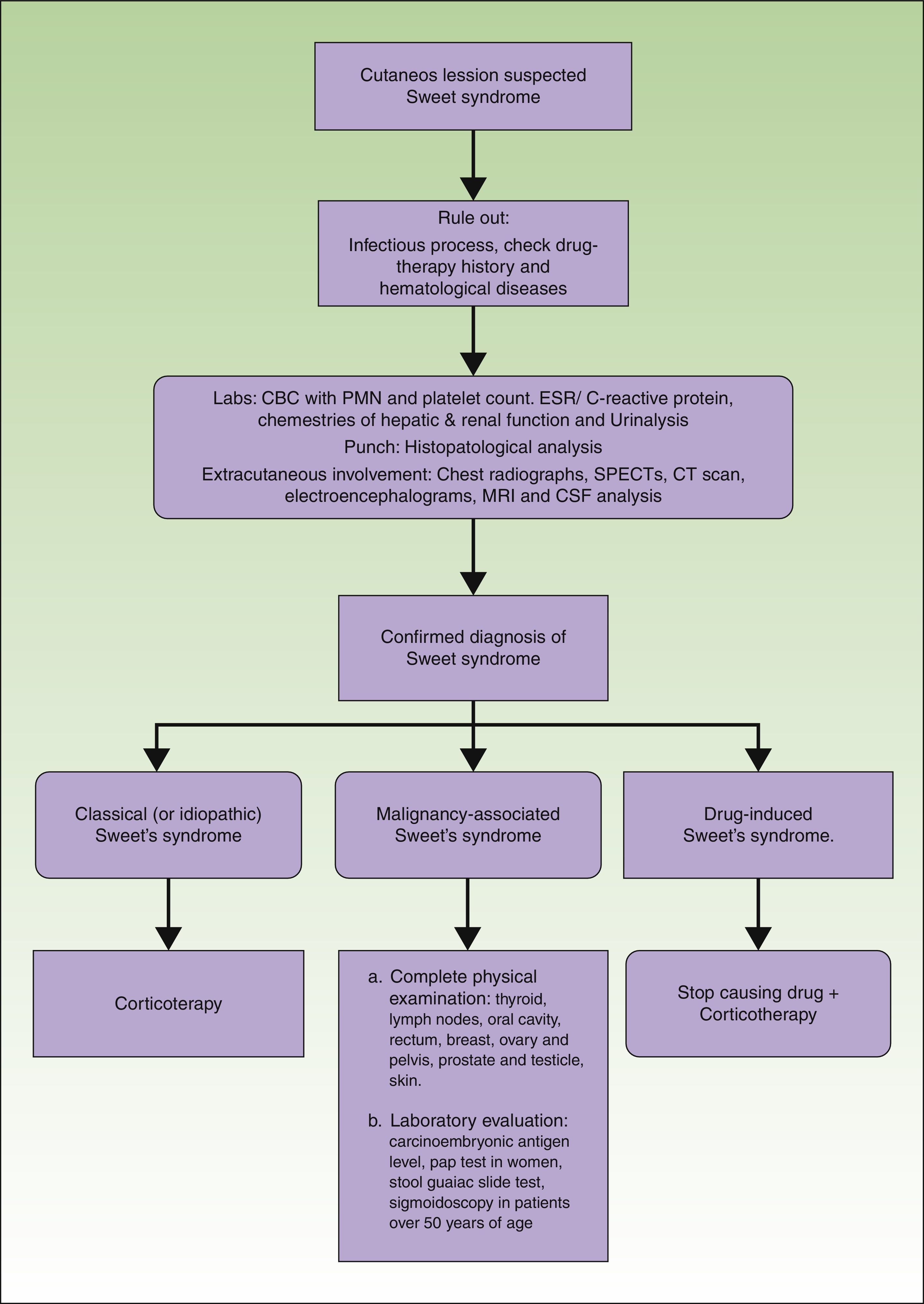
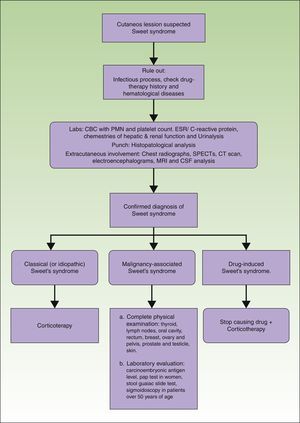
ManagementThe management of patients with a Sweet's syndrome can be performed in 3 steps: assessment, workup, and treatment. Assessment includes the identification of the type of cutaneous lesion, the existence of possible extracutaneous sites, and the search for associated disease. In every case, it is important to rule out the presence of an infection and to have a precise drug-therapy history. Also, the potential presence of hematological diseases must be systematically investigated. Furthermore, a routine or targeted search for cancer is performed according to the age and symptoms of the patient. In the absence of symptoms, aggressive investigatory procedures are unwarranted.
Laboratory evaluation should include a complete blood cell count with leukocyte differential and platelet counts. Evaluation of acute phase reactants, including the erythrocyte sedimentation rate or C-reactive protein, serum chemistries for evaluating hepatic and renal function, and a urinalysis should also be performed. It may also be reasonable to perform a serologic evaluation for antistreptolysin-O antibody, rheumatoid factor, and thyroid function because streptococcal infection, rheumatoid arthritis, and thyroid disease have been found to have either a probable or bona fide association with Sweet's syndrome.3 Although the most consistent laboratory abnormalities in Sweet's syndrome are peripheral leukocytosis with neutrophilia and an elevated erythrocyte sedimentation rate,7 it is not always observed in all patients with biopsy-confirmed Sweet's syndrome.21 For example, some of the patients with malignancy-associated Sweet's syndrome may present with anemia, neutropenia, and/or abnormal platelet counts. A work-up diagram is presented in Fig. 3.
A lesional skin biopsy for routine histopathologic evaluation is a useful procedure to confirm a clinically suspected diagnosis of Sweet's syndrome. A 4mm wide punch can be performed in the most recent skin lesion. The skin biopsy should present the pathologic features of Sweet's syndrome, such as the diffuse inflammatory infiltrate of neutrophils in the dermis, subcutaneous fat, or both, which can also be observed in cutaneous lesions caused by an infectious agent. Therefore, it may also be prudent to submit lesional tissue for bacterial, fungal, mycobacterial, and possibly viral cultures.3
If extracutaneous involvement of Sweet's syndrome needs to be ruled out, chest radiographs, SPECTs, computerized axial tomography, electroencephalograms, magnetic resonance imaging and even cerebrospinal fluid analysis can be performed, depending on the suspected location. For instance, urinalysis abnormalities, such as hematuria and proteinuria, may be observed in patients with kidney involvement, and hepatic serum enzyme elevation may be present in patients with Sweet's syndrome-associated liver involvement. Pleural effusions and corticosteroid-responsive culture-negative infiltrates may be present on chest roentgenograms in patients with Sweet's syndrome who have extracutaneous manifestations that involve their lungs.47
Recommendations for the initial malignancy workup in newly diagnosed Sweet's syndrome patients without a prior cancer diagnosis were proposed by Cohen and Kurzrock in 1993.17 Their recommendations are based on the neoplasms that were concurrently or subsequently discovered in previously cancer-free Sweet's syndrome patients and on the age-related recommendations of the American Cancer Society for the early detection of cancer in asymptomatic persons.48 They recommended the obtaining the following: detailed medical history; complete physical examination, including an examination of the thyroid, lymph nodes, oral cavity, and skin, digital rectal examination, breast, ovary, and pelvic examination in women, and prostate and testicle examination in men; laboratory evaluation, including carcinoembryonic antigen levels, complete blood cell count with leukocyte differential and platelet count, pap test in women, serum chemistries, stool guaiac slide test, urinalysis, and urine culture; and other screening tests such as chest roentgenograms, endometrial tissue sampling in either menopausal women or women with a history of abnormal uterine bleeding, estrogen therapy, failure to ovulate, infertility, or obesity, and sigmoidoscopy in patients over 50 years of age. Cohen and Kurzrock also suggest performing a complete blood cell count with leukocyte differential and platelet count every 6–12 months because the initial appearance of dermatosis-related skin lesions may precede the diagnosis of a Sweet's syndrome-associated hematologic malignancy by as much as 11 years.3
Sweet's syndrome lesions, without any therapeutic intervention, can remain for weeks to months but eventually resolve in some patients with classical Sweet's syndrome.2 In malignancy associated Sweet's syndrome, the remission of the related cancer is occasionally followed by resolution of the dermatoses. In cases of drug-induced Sweet's syndrome, improvement and subsequent clearing of the syndrome occurs after stopping the associated medication.7
Although there are no guidelines for the treatment of Sweet's syndrome, systemic corticosteroids are the first-line of treatment in most cases; oral therapy with either potassium iodide or colchicine in patients for whom corticosteroids are contraindicated also typically results in the rapid resolution of Sweet's syndrome symptoms and lesions.49 Cutaneous and extracutaneous manifestations tend to improve within the first 72h of the start of therapy.50 Sweet's syndrome can be treated initially by general corticotherapy with prednisone or with an initial prednisone dosage of 30–60mg/day (0.5–1.5mg/kg/day), with subsequent gradual reduction.7,51 In localized lesions, high-potency topical corticosteroids or intralesional corticosteroids may be used.7 Alternative treatments such as potassium iodide tablets (900mg/day) or colchicine solution (1.5mg/day) are also considered first-line agents.
Second-line systemic therapies include indomethacin (50–150mg/day), clofazimine (100–200mg/day), dapsone (100–200mg/day) and cyclosporine (2–4mg/kg/day).7 Other drugs prescribed for treatment include doxycycline, metronidazole, etretinate, chlorambucil, cyclophosphamide, methotrexate, etanercept, infliximab and thalidomide. The efficacy of IL-1 blocking agents such as anakinra in refractory cases has been recently published.52
In conclusion, Sweet's syndrome is a complex global disease without racial disparity that occurs more frequently in middle age women. Although the etiology of Sweet's syndrome is not completely understood, it has an inflammatory component in all 3 of its varieties. Importantly, this syndrome could be the first manifestation of a malignancy, so each case should include an individual work-up. Corticosteroids are the main line of treatment, as recurrence is very common. Follow-up is crucial. Although there is no consensus in the literature and the recommendations range from simple monitoring to follow-up care, we should always endeavor to keep well informed of patient conditions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.