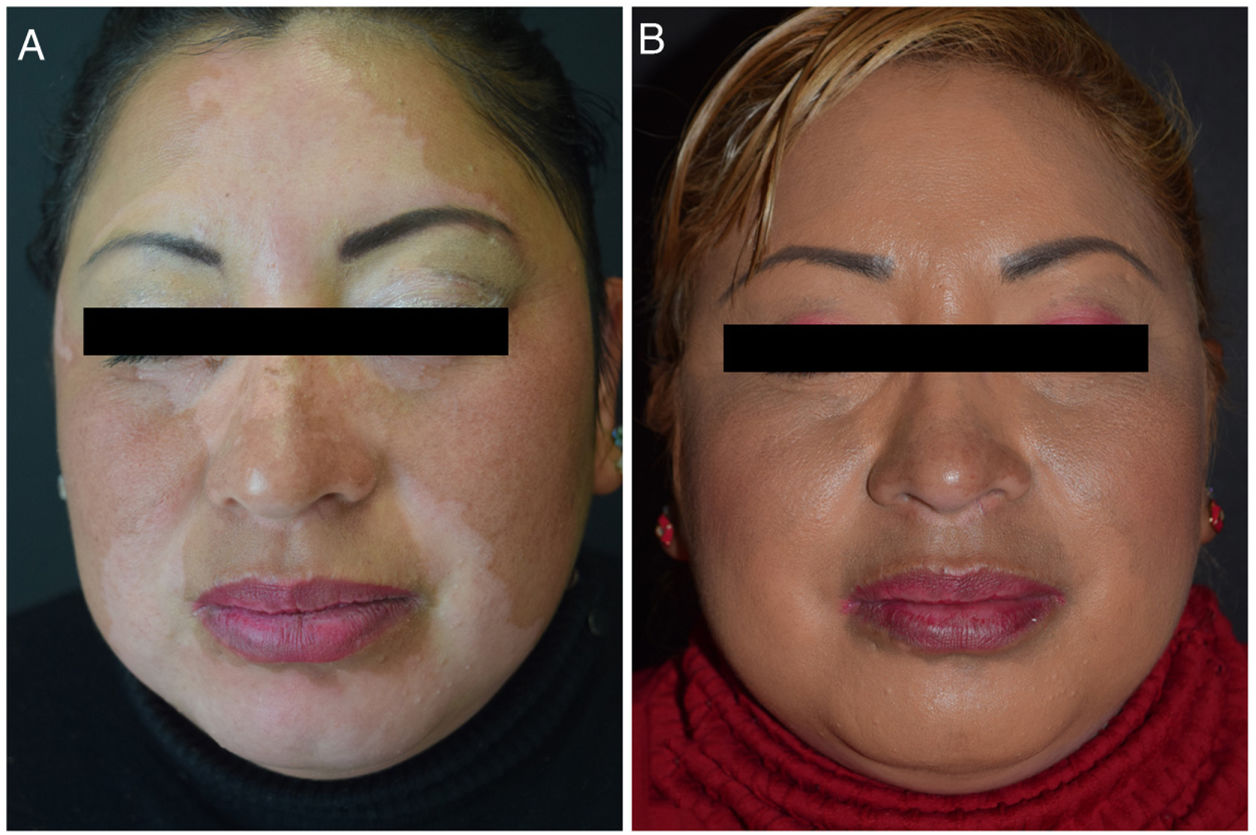
Vitiligo is the most common acquired form of skin dyschromia and impacts the quality of life of affected individuals.1,2 Cosmetic camouflage is a therapeutic alternative that can be used to improve skin appearance and hence quality of life in patients with skin dyschromia.3 Several studies have demonstrated the effectiveness of this approach.4,5
An uncontrolled quasi-experimental study of patients with facial vitiligo treated at the Dr. Ladislao de la Pascua dermatology center, Mexico City, between July 2017 and May 2019, evaluated the impact of cosmetic camouflage on patient quality of life using generic and specific scales. The study was approved by relevant ethics and research committees and all study participants provided written informed consent. Adult vitiligo patients with spots in the facial region were recruited, regardless of the clinical variety and extent of the disease. Those with other concomitant dermatological diseases affecting the face, psychiatric diseases (depression and anxiety), and/or allergies to cosmetics were excluded.
Quality of life was assessed using disease-specific (vitiligo quality-of-life index [VitiQol]) and generic (Dermatology Life Quality Index [DLQI], EuroQol-5 Dimension [EQ5D], and the Short Form Health Survey [SF36]) scales at the initial visit (V1) and at weeks 8 (V2) and 16 (V3) of the study. Validated Mexican Spanish versions of all scales were used. For normally distributed data, a repeated measures analysis of variance (ANOVA) test was used to compare the scores of the quality-of-life questionnaires. In all other cases, the Friedman test was used. Statistical significance was set at P<0.05.
Participants were instructed how to use cosmetic camouflage in a workshop led by a professional makeup artist. The product used was Dermablend (Vichy Laboratories). The authors received no financial compensation for the use of this protocol and Vichy México was not involved in the study design or execution, or the statistical analysis. All participants were instructed to apply cosmetic camouflage for 16 weeks.
A total of 45 vitiligo patients with facial involvement were recruited. Of these, 42 completed 2 study visits and 38 completed the 3 scheduled visits. The sociodemographic variables of the study population are shown in Table 1. Significant changes in post- versus pre-intervention quality-of-life scores in the different questionnaires are shown in Table 2. In the DLQI questionnaire, a significant change in the effect of vitiligo on quality of life was observed for 27 (64.3%) patients: quality-of-life improvements were observed in 1 category for 18 (66.7%) patients, 2 categories for 6 (22.2%) patients, and 3 categories for 3 (11.1%) patients. These findings were similar to those of Ogenage et al.4 In our study, the use of cosmetic camouflage was associated with a positive change in quality of life that was evident within the first 8 weeks of intervention and, according to the results of the post hoc analysis, persisted until the end of the study (except for some categories of the SF36 questionnaire). These findings reflect a change that occurred shortly after beginning the intervention, as reported in similar studies in pediatric populations.6 The specific scale used to measure quality of life in our study was the ViTiQol score, for which significant differences were observed after versus before the intervention. Our study is the first to use this instrument to evaluate the effect of interventions in vitiligo patients and revealed a significant correlation (P<0.001) between the DLQI and ViTiQol questionnaire findings, in line with a previous validation study performed in a Mexican population.7
Characteristics of the Participants.
| Variable n (%) | |
|---|---|
| Sex | |
| Male | 10 (22.2%) |
| Female | 35 (77.8%) |
| Age* | 42.4±13.7 y |
| Education level | |
| No formal schooling | 1 (2.2%) |
| Lower | 12 (26.7%) |
| Medium | 21 (46.7%) |
| Higher | 11 (24.4%) |
| Occupation | |
| Worker | 24 (53.3%) |
| Non-worker | 21 (46.7%) |
Pre- Versus Post-Intervention Questionnaire Results.
| Before | After | P | |
|---|---|---|---|
| ViTiQola | 46.2±22.7 | 31.9±20.4 | <0.001 |
| DLQIb | 8 (3.5-12) | 3 (1-6.25) | <0.001 |
| EQ5D (visual analog scale) | 82 (80-90) | 90 (83-95) | 0.007 |
| SF36b | |||
| Emotional limitations | 100 (33.3-100) | 100 (100-100) | 0.017 |
| Emotional well-being | 68 (52-78) | 76 (55-84) | 0.011 |
| Social functioning | 75 (62.5-100) | 100 (75-100) | 0.023 |
Abbreviations: DLQI, Dermatology Life Quality Index; EQ5D, EuroQol-5 Dimension questionnaire; SF36, Short Form Health Survey; ViTiQol, Vitiligo Quality-Of-Life Index.
Analysis of the other study variables (sex, age, educational level, and occupation) revealed no significant differences that were maintained at the end of the study.
In conclusion, despite the study's limitations (including a predominantly female study population, the lack of a control group, and the absence of comparison with another intervention to improve quality of life), our findings demonstrate an improvement in quality of life in vitiligo patients who used cosmetic camouflage (Fig. 1). This intervention proved to be fast and safe, with adequate adherence and good patient satisfaction, and therefore represents a valid option as a therapeutic supplement for vitiligo.
FundingDermablend products were donated by Vichy México. The authors did not receive any financial compensation for the study protocol used. Vichy México was not involved in the study design or execution, or in the statistical analysis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.