When co-administered with interferon and ribavirin, the prescription drug telaprevir significantly improves treatment response in patients with chronic hepatitis C virus (HCV) infection. Its use, however, also increases the likelihood of adverse effects that may lead to discontinuation of treatment. Cutaneous adverse effects are particularly common.
ObjectiveTo determine the frequency and clinical characteristics of drug eruptions induced by telaprevir in patients receiving HCV treatment and to analyze the clinical course of lesions and response to treatment.
Material and methodsWe performed a prospective observational study of all patients who started a treatment regimen that included telaprevir between May 2012 and July 2013. We recorded the demographic characteristics of the patients who developed telaprevir-induced eruptions, and analyzed the clinical characteristics of the lesions and their clinical course following the application of guideline-based treatment recommendations.
ResultsTwenty (46%) of the 43 patients who received triple therapy with interferon, ribavirin, and telaprevir during the study period developed drug reactions attributable to telaprevir. The reaction was classified as mild or moderate (grades 1 or 2) in 90% of cases and consisted of an exanthem with erythematous-edematous scaling plaques and papules. The rash worsened, mainly by spreading, in about one-third of cases. The skin lesions led to discontinuation of treatment in 2 patients (4.6%). Sustained viral response was achieved in 34 patients (79%).
ConclusionsTelaprevir-induced eruptions are common and often progress, but they rarely require patients to discontinue treatment.
Telaprevir es un fármaco que administrado junto a interferón y ribavirina incrementa de forma significativa la respuesta al tratamiento de la infección por el virus de la hepatitis C. Sin embargo, su empleo incrementa también la probabilidad de desarrollar efectos adversos, en muchos casos cutáneos que pueden condicionar el mantenimiento del tratamiento.
ObjetivoConocer la incidencia, características clínicas y evolutivas y respuesta al tratamiento de las toxicodermias por telaprevir en el contexto del tratamiento de la infección por el virus de la hepatitis C.
Material y métodosEstudio prospectivo observacional realizado entre mayo de 2012 y julio de 2013 en el que se incluyeron aquellos pacientes que iniciaron tratamiento con telaprevir durante ese periodo. En aquellos en los que se detectaron toxicodermia se recogieron los datos demográficos de los pacientes, las características clínicas de las lesiones y la evolución tras la aplicación de las recomendaciones de las guías clínicas.
ResultadosDe un total de 43 pacientes que recibieron tratamiento triple un 46% presentó toxicodermia atribuible a telaprevir. En el 90% de los casos esta fue leve o moderada (grados 1 o 2) y consistió en un exantema constituido por pápulas y placas eritematoedematosas y descamativas. En alrededor de un tercio de los pacientes se comprobó la progresión de la toxicodermia, principalmente en extensión, durante el curso del tratamiento. En 2 casos (4,6%) las lesiones cutáneas condicionaron la suspensión del fármaco. Un 79% de los tratados (34 pacientes) alcanzó una respuesta viral sostenida tras el tratamiento.
ConclusionesLas toxicodermias asociadas a telaprevir son frecuentes en el curso del tratamiento y a menudo progresivas. Sin embargo, solo de forma excepcional condicionan su suspensión.
In developed countries, chronic hepatitis C virus (HCV) infection is currently the main cause of cirrhosis, hepatocellular carcinoma, and death from liver-related diseases, as well as a leading indication for liver transplant.1 Standard treatment, which is based on the combination of pegylated interferon and ribavirin, provides modest rates of sustained virological response (SVR), defined as the absence of viral load in blood 24 weeks after completion of treatment. Since achieving SVR has been associated with a clear improvement in the prognosis of the underlying liver disease and survival,2 it is considered the main objective of treatment of HCV infection. During the last few years, efforts to develop new agents to improve the treatment of patients with this disease have yielded a new drug class known as direct-acting antiviral agents.
In 2011, the United States Food and Drug Administration and the European Medicines Agency approved telaprevir for the treatment of chronic genotype 1 HCV infection.3 Telaprevir is a direct-acting antiviral agent that rapidly reduces HCV RNA levels by binding to the protease serine NS3/4.A, an enzyme that is essential for viral replication.4 When telaprevir is administered with interferon and ribavirin (triple therapy), a significant increase is observed in the SVR rate in patients infected by HCV genotype 1.5 Thus, the SVR rate achieved with telaprevir reaches 75% in previously untreated patients6 and up to 70% in previously treated patients7 compared with more modest rates of around 50% for pegylated interferon and ribavirin.8
The recommended treatment regimen is 12 weeks with triple therapy followed by 12–36 weeks of interferon and ribavirin depending on the viral response and the presence or absence of cirrhosis.
However, adding telaprevir to standard HCV therapy increases the risk of adverse events, among which skin complaints are common. In addition, patients who receive telaprevir suspend treatment more often than those who take standard treatment.9
Although telaprevir-associated skin manifestations have been reported in clinical trials and guidelines for management of these manifestations have been drafted, we know little of the incidence, magnitude, clinical expression, and outcome of drug eruptions in daily practice.
Prospective assessment of telaprevir-associated cutaneous adverse events could favor rapid recognition and management. Therefore, we designed the present study to ascertain the incidence of cutaneous adverse reactions associated with triple therapy for HCV infection. We also analyzed clinical characteristics, time of onset after initiation of treatment, outcome, and response to treatment.
Material and MethodsWe performed a prospective observational study of all patients at Hospital Universitari Germans Trias i Pujol, Badalona, Spain who were diagnosed with infection by HCV genotype 1 and who started triple therapy (telaprevir, interferon, and ribavirin) between May 2012 and July 2013.
Before antiviral treatment was started, the patient attended a baseline visit at the dermatology clinic, where pre-existing skin conditions were recorded, as were previous anti-HCV therapy with interferon and ribavirin and the presence of cutaneous adverse events associated with the regimen. At this visit, the patient was provided with recommendations for regular care and hydration of the skin during treatment with telaprevir. In addition, the need to consult quickly for skin lesions during antiviral therapy was stressed.
All patients underwent checkups at the liver unit of our hospital. At the visits, tolerance to treatment was assessed, and serial analyses were performed to evaluate both the effectiveness of therapy (SVR) and the onset of anemia and other disorders. In the case of skin lesions, patients were sent to the dermatology department within 24–48hours.
The role of telaprevir in the eruptions was assessed using the causality algorithm of Naranjo et al.10
In cases that were clinically compatible with drug eruption, the disease was classified based on the proposal shown in the Ministry of Health guidelines on treatment of HCV infection with triple therapy (Table 1).11 This evaluation was accompanied by a detailed description of the skin lesions and images. The Ministry of Health document was also taken into account as a reference for therapy guidelines.
Classification of Telaprevir-Induced Skin Eruptions According to Severity and Recommended Protocol in Phase III Clinical Trials.
| Classification | Clinical Characteristics | Recommended Protocol |
|---|---|---|
| Grade 1 (mild) | Localized skin rash with(out) associated pruritus | Hydration. Limit exposure to sunlight/heatEmollient creams/lotionsTopical corticosteroidsSystemic antihistamines |
| Grade 2 (moderate) | Diffuse skin rash affecting at least 50% of body surface with(out) scaling, pruritus, or mucosal involvement and no ulceration | Same as grade 1Suspend telaprevir if condition worsensSuspend ribavirin if no improvement after 7 days |
| Grade 3 (severe) | Exanthem affecting more than 50% of body surface or exanthem that presents as vesicles or blistering, superficial ulceration of mucosa, or skin detachment | Suspend telaprevir immediatelySuspend ribavirin and then interferon if no improvement after 7 days |
| Grade 4 (SCADR) | Stevens-Johnson syndromeDRESS syndromeToxic epidermal necrolysisErythema multiformeGeneralized exanthematous pustulosis | Permanent suspension of all drugs (telaprevir, ribavirin, and interferon)Consider hospitalization |
Abbreviations: DRESS, drug rash with eosinophilia and systemic symptoms; SCADR, severe cutaneous adverse drug reactions.
In all cases of drug eruption, we designed a personalized follow-up schedule with individual checkups in the dermatology and hepatology departments. The checkups continued until either the skin complaint resolved or until it was recommended to suspend telaprevir. In cases where the clinical picture persisted after suspension of telaprevir, the patient was to continue having checkups in the dermatology department to evaluate the sequential withdrawal of ribavirin and interferon.
ResultsWe included 43 patients (29 men, 14 women) aged between 27 and 67 years. Of all the patients studied, 16 (37%) had a history of pre-existing skin complaints: 6 patients had seborrheic dermatitis, 3 capillaritis, 3 psoriasis, 2 urticaria, 1 atopic dermatitis, and 1 lichen planus. Twenty-five patients (58%) had received standard antiviral therapy (Table 2). Of these 25 patients, 6 (24%) had experienced skin reactions—all mild—to treatment with interferon and ribavirin.
Twenty patients (46%) had a skin eruption considered to be associated with triple therapy. The age, sex, and other demographic characteristics of these 20 patients are shown in Table 3.
Clinical Characteristics of Patients With Telaprevir-Induced Skin Eruption.
| Patient | Age | Sex | Previous Anti-HCV Therapy | Grade of Drug Eruption | Treatment Administered | Course of Skin Condition | Virological Course |
|---|---|---|---|---|---|---|---|
| Patient 1 | 52 | M | No | Grade 1 | Topical | Cured | SVR |
| Patient 2 | 49 | M | Yes | Grade 1 | Topical | Cured | No SVR |
| Patient 3 | 64 | M | No | Grade 1 | Topical | Cured | SVR |
| Patient 4 | 61 | W | No | Grade 2 | Topical and oral corticosteroids | Progression to grade 3a | SVR |
| Patient 5 | 44 | M | No | Grade 1 | Topical | Cured | No SVR |
| Patient 6 | 63 | M | Yes | Grade 1 | Topical | Cured | SVR |
| Patient 7 | 57 | M | No | Grade 1 | Topical | Cured | SVR |
| Patient 8 | 51 | M | Yes | Grade 1 | Topical | Progress to grade 2 then cured | SVR |
| Patient 9 | 34 | W | No | Grade 1 | Topical | Cured | SVR |
| Patient 10 | 55 | M | Yes | Grade 1 | Topical | Cured | SVR |
| Patient 11 | 58 | W | No | Grade 2 | Topical | Progress to prurigo nodularis–type lesionsb | SVR |
| Patient 12 | 57 | W | No | Grade 4 | Immediate suspension of telaprevir | Cured | No SVR |
| Patient 13 | 63 | W | No | Grade 1 | Topical | Progress to grade 2 then cured | SVR |
| Patient 14 | 62 | M | No | Grade 2 | Topical | Cured | SVR |
| Patient 15 | 65 | W | No | Grade 1 | Topical | Cured | SVR |
| Patient 16 | 62 | M | Yes | Grade 1 | Topical | Cured | SVR |
| Patient 17 | 42 | W | No | Grade 1 | Topical | Cured | SVR |
| Patient 18 | 65 | W | No | Grade 1 | Topical | Cured | SVR |
| Patient 19 | 51 | M | Yes | Grade 1 | Topical | Cured | SVR |
| Patient 20 | 62 | M | No | Grade 1 | Topical | Cured | SVR |
Abbreviation: SVR, sustained virological response.
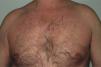
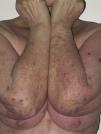
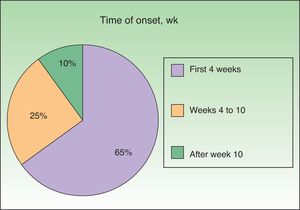
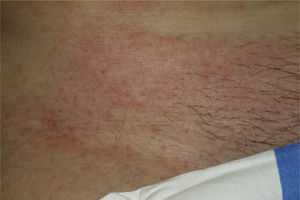
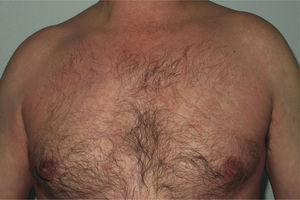
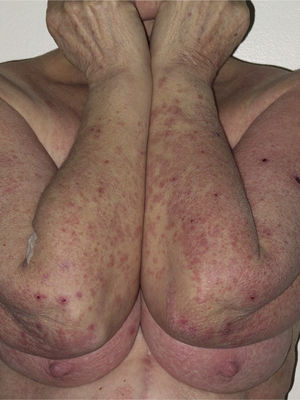
Onset was quick (within the first 4 weeks of treatment) in most eruptions (13/20) (Fig. 1). The lesions presented mainly as an exanthem consisting of erythematous-edematous scaling papules and plaques that were poorly delimited and small. The lesions were initially localized before extending more or less symmetrically, mainly across the upper part of the body (Fig. 2) but sparing the face and the soles and palms. The lesions were almost always associated with pruritus, cutaneous xerosis, and signs of excoriation. Involvement of the oral mucosa (minute erosions) was detected in only 1 case.
In order to rule out other skin processes and establish a correlation between clinical and histopathologic findings, we performed a skin biopsy in 7 patients. Most biopsy specimens (6/7) showed a pattern of spongiotic or interface dermatitis, which was associated with a superficial and deep perivascular infiltrate containing eosinophils. In 1 case, we observed a pattern of leukocytoclastic vasculitis characterized by a predominantly polymorphonuclear perivascular infiltrate of the walls and vessels and the presence of leukocytoclasia.
Most of the eruptions (16/20) were initially classed as mild (grade 1). However, in one-third of cases (33%), the lesions progressed during follow-up to more severe or extensive forms, with an increase in the area of skin affected (Figs. 3 and 4). Thus, 6 patients progressed from grade 1 to grade 2 and 1 patient progressed from grade 2 to grade 3.
As for treatment, 19 of the 20 patients who experienced eruptions (95%) received middle-potency topical corticosteroids combined with emollients. Patients who received topical corticosteroids always applied them until their skin complaint resolved. In the case of pruritus, patients were also prescribed oral antihistamines according to the management guidelines.
The recommended regimen for telaprevir was completed by all but 2 patients (4.6% of all those treated). In the first case, treatment was suspended because of extensive cutaneous vasculitis that appeared shortly after the start of treatment. In the second case, telaprevir was suspended almost at the end of week 11 because the lesions progressed from grade 2 to grade 3, although this did not alter the effectiveness of antiviral therapy. Neither patient had previously received antiviral drugs or had a history of skin complaints. None of the 20 patients diagnosed with drug eruption had to be admitted to hospital.
Finally, SVR was achieved after 24 weeks of antiviral therapy in 34 of the 43 patients studied (79%).
DiscussionAlmost half of the patients treated with triple therapy in our study had symptoms compatible with drug eruption; this percentage was similar to those reported elsewhere in the literature. In most cases, the eruption was mild or moderate and, despite progressing in 33% of cases, was controlled with adjuvant therapy. It was only necessary to suspend treatment in 2 cases.
In contrast with studies reporting that these skin reactions can appear at the start and end of triple therapy,12 we found that the vast majority were early, occurring within the first 4 weeks of starting treatment. It seems likely that the prospective design of our study, with close and proactive follow-up of the patient, made it easier to recognize the eruptions.
Although it has been demonstrated that, in the context of triple therapy, more than 90% of skin reactions are caused by telaprevir, it is important to remember that interferon and ribavirin may also be associated with cutaneous adverse effects, which are often indistinguishable from those caused by telaprevir.13 We evaluated the patients in our study at baseline. In addition to providing a setting where we could provide the patient with recommendations for regular skin care and hydration during triple therapy and encourage patients to consult quickly in the event of lesions appearing, the baseline visit served to reveal the presence of previous skin complaints that could imply a greater risk of drug eruption or that could be confused with drug eruption. It also enabled us to investigate any history of drug eruption caused by anti-HCV treatment in previously treated patients. Thus, at the baseline visit, we learned that 25 patients (58%) had previously received interferon and ribavirin and that 6 of these patients (24%) had had a skin reaction, which was mild in all cases.
In any case, neither the presence of pre-existing skin complaints nor a previous history of drug eruption entailed a greater risk of skin reactions caused by triple therapy in our series.
Telaprevir-induced skin reactions are recorded in approximately half of all patients treated with the drug.14 The mechanism underlying the reactions is unknown, and studies performed to date have been unable to show genetic susceptibility or pharmacokinetic variations that make reactions more likely. The specific clinical characteristics that are considered risk factors include age over 45 years, obesity, white ethnicity, and no previous treatment.15
Although most cases of telaprevir-induced eruption are mild or moderate, severe (and potentially fatal) reactions have been reported.16 Consequently, a protocol for the diagnosis and management of telaprevir-induced eruption was drafted to minimize the effects of this condition during treatment. Drug-induced eruption was classified by intensity, which is mainly—although not exclusively—based on the percentage of body surface affected (Table 1).
In our series, most cases of drug eruption involved an erythematous eruption in the form of scaling and erythematous-edematous plaques and macules. Of the total number of cases of drug eruption, 14 initially involved grade 1 lesions, 5 grade 2 lesions, and 1 grade 3 lesions (Table 3). These figures are consistent with those reported elsewhere. In terms of histopathology, most of the biopsied lesions (6 of 7) had a microscopic pattern consistent with drug eruption, even though the diagnosis of drug eruption is essentially based on clinical symptoms.
Furthermore, in contrast with reports from the literature stating that drug eruption was stable with no progression to severity until the end of treatment with telaprevir in almost 90% of cases,12 we found that 33% of patients with skin complaints progressed to more severe grades, requiring treatment to be suspended in 1 case. Consequently, as is usual in adverse drug reactions, we can see that telaprevir-induced eruption should be understood as a dynamic process that can evolve over time and in which the clinical characteristics of skin lesions are more important than the extension of the lesions. In this sense, classification associating severity with extension could prove to be somewhat inadequate for prognostic purposes, as recently proposed by Rojeau et al.15
In almost all cases, treatment was with moderately potent topical corticosteroids, which were almost always applied in combination with emollients. Oral antihistamines were added in the case of pruritus. Systemic corticosteroids were only prescribed in the 2 cases in which telaprevir was suspended early, but not in the remaining cases, since it has been reported that administration of these drugs with triple therapy can alter the efficacy of telaprevir and increase viral RNA levels as a result of the interaction between the drugs in the CYP3A4 liver metabolic pathway.17 However, Garcias-Ladaira et al.18 reported prescription of prednisone in extensive eruptions that had not responded to topical medication, with no interaction affecting the virological response, thus enabling the triple therapy regimen to be completed.
We found telaprevir to be an efficient and safe drug, with a high percentage of SVR (79%), a small frequency of severe adverse reactions (4.9%), and no grade 4 cutaneous reactions.
Although our study was prospective, it is subject to a series of limitations that are inherent to its design. First, it was performed in a hospital clinic and included a low number of patients. Second, patients were monitored closely and proactively, in contrast with the usual follow-up of patients who undergo treatment for HCV infection.
The development of joint protocols by dermatologists and hepatologists meant that patients were diagnosed and treated quicker. Therefore, differences with other publications when the lesions appeared and the progressive character of the lesions could reflect earlier recognition and better monitoring of skin lesions.
In conclusion, adverse cutaneous reactions to telaprevir are common during triple therapy and almost always present as scaling and erythematous-edematous maculopapular exanthems. They often appear early and progress over the following weeks. The treatment recommended in clinical guidelines seems appropriate for control of the reactions, and only exceptionally is it necessary to suspend treatment. Dermatological monitoring is essential to ensure suitable management of telaprevir-induced skin reactions.19
Ethical DisclosuresProtection of persons and animalsThe authors declare that this research did not involve experiments performed on humans or animals.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Toro Montecinos M, Carrascosa Carrillo JM, Vilavella Rius M, Bielsa Marsol I, Plana Pla A, Morillas Cunill R, et al. Toxicodermias por telaprevir en el tratamiento de la infección crónica por el genotipo 1 del virus de la hepatitis C. Estudio prospectivo. Actas Dermosifiliogr. 2015;106:219–225.