We report a case of angiomatoid melanoma on the right thigh of a 59-year-old man. The histologic growth pattern of the tumor mimicked vascular proliferation, and the cells lining the pseudovascular spaces were positive for protein S-100, HMB-45, and MITF-1. The differential diagnosis is with angiosarcoma and pseudovascular adenoid squamous cell carcinoma. The case we present is the fifth reported to date.
Presentamos un caso de melanoma angiomatoide localizado en la piel del muslo derecho en un hombre de 59 años de edad. La neoplasia mostró un patrón de crecimiento semejante a una proliferación vascular donde las células que revestían esos espacios “pseudovasculares” fueron positivas a la proteína S-100, al HMB45 y al MiTF1. El diagnóstico diferencial incluye el angiosarcoma y el carcinoma escamoso pseudovascular. El caso que aquí informamos es el quinto de la literatura mundial.
Melanoma has diverse morphologic forms. Among the most common forms are superficial spreading melanoma, nodular melanoma, acral lentiginous melanoma, desmoplastic melanoma, and lentigo maligna melanoma. Some of the rarer forms, which fall into the category of miscellaneous melanomas, are melanomas with rosettes, angiotropic melanoma, animal-type melanoma, myxoid melanoma, chondroid melanoma, osteogenic melanoma, rhabdoid melanoma, follicular melanoma, nevoid melanoma, and angiomatoid melanoma. Angiomatoid melanoma was first described in metaplastic melanomas with clusters of neoplastic cells reminiscent of vascular channels. Immunohistochemically, this variant of melanoma is negative for vascular markers and positive for melanocytic markers. The angiomatoid pattern has been described in just 4 cases of melanoma to date in the English literature. The differential diagnosis is with angiosarcoma and pseudovascular squamous cell carcinoma.1
We describe a case of primary angiomatoid melanoma on the right thigh of a 59-year-old man, with no evidence of metastasis at the time of diagnosis.
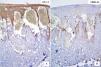
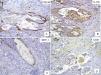
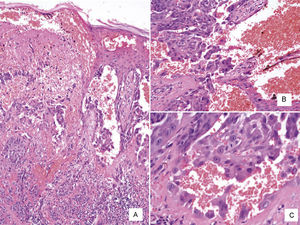
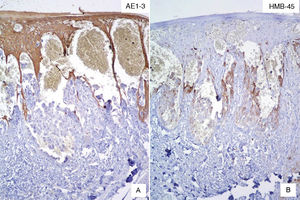
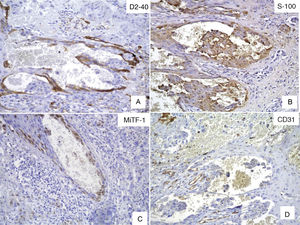
Case DescriptionWe present the case of a 59-year-old man with a skin lesion on his right thigh. The patient reported that the lesion had been present for several months, but he did not specify the exact time. The clinical diagnosis was “probable hemangioma” and the lesion was completely excised. Histologic examination showed a nodular neoplastic lesion with infiltrating margins in the epidermis, extending into the reticular dermis. The neoplastic cells were medium to large in size and had pronounced pleomorphism, with several cells with irregular nuclei, granular chromatin, prominent nucleoli, and abundant cytoplasm interspersed with smaller cells with hyperchromatic nuclei and amphophilic cytoplasm (Fig. 1). Also identified was dispersed melanic pigment that was positive with Fontana-Masson stain. There were 2 mitoses in 10 high-power fields (×40). The most striking finding was the presence of large channels with a cavernous vascular appearance filled with erythrocytes and surrounded by neoplastic cells (Fig. 1). Immunohistochemical staining of the neoplastic cells was positive for S-100 protein, HMB-45, and MiTF-1, and uniformly negative for CD31 (Figs. 2 and 3). The cells were also positive for D2-40 (podoplanin) and negative for keratin AE1 and AE3 and p63. The Ki-67 proliferation index was 5% to 10%. Based on the above findings, a diagnosis of angiomatoid nodular melanoma was established, with a Clark level of IV, a Breslow thickness of 1.35mm, absence of vertical growth phase, a moderate lymphocytic infiltrate at the base, no evidence of vascular or perineural invasion, or microsatellitosis, and tumor-free lateral and deep margins.
Alder et al.2 published the first description of angiomatoid melanoma in 1997. They reported the case of a 44-year-old man with melanoma metastasis to the forehead skin and sacral vertebra. The origin of the primary tumor was unknown. The metastasis on the forehead showed clusters of cells forming cavernous spaces containing numerous erythrocytes and lined by neoplastic cells that stained positive for HMB-45 and S-100 protein and negative for vascular markers. The authors proposed the name angiomatoid melanoma to describe this variant of melanoma. Three additional cases have since been reported3,4 (Table 1).
Cases of Angiomatoid Melanoma in the Literature.
| Age, y | Sex | Location | Diagnosis | Immunohistochemistry | Reference | |
|---|---|---|---|---|---|---|
| 1 | 44 | Male | 1) Intravertebral (S1-S2)2) Forehead | 1) Melanoma2) Angiomatoid metastatic melanoma | 1) S-100+, HMB-45+, vimentin+2) S-100+, HMB-45+, vimentin+ | 2 |
| 2 | 84 | Male | Periorbital region | Desmoplastic and amelanotic melanoma with an angiomatoid pattern | S100+ | 3 |
| 3 | 56 | Female | 1) Right arm2) Back (paravertebral muscles), lower left quadrant of the left breast, neck, subcutaneous tissue (right shoulder, psoas muscle, medial gluteus) | 1) Melanoma, Clark level III, Breslow thickness 1.1mm2) Angiomatoid metastatic melanoma | 1) S-100+, Melan A+, HMB-45+2) S-100+, HMB-45+, Melan A+, CD56+ | 4 |
| 4 | 61 | Male | 1) Third finger of the left hand2) Axillary lymph node | 1) Acral lentiginous melanoma, Clark level III, Breslow thickness 0.55mm2) Angiomatoid metastatic melanoma | 1) S-100+2) S-100+, CD56+ | 4 |
| 5 | 59 | Male | Right thigh skin | Angiomatoid nodular melanoma | S-100+, HMB 45+, MiTF-1+Keratin AE1/AE3–, CD31– | Current case |
Pseudovascular spaces have been described in benign pigmented nevi, and attributed to artifacts of tissue processing or trauma during the biopsy procedure.5 Alterations in elastic fibers and/or collagen in the nevi could reduce the resistance of the dermis to the mechanical stress of the biopsy procedure, leading to the formation of these spaces.5
In a study of invasive uveal melanomas with pseudovascular spaces, Maniotis et al.6 demonstrated the absence of endothelial cells by light microscopy, transmission electron microscopy, and immunohistochemistry. They also demonstrated in vitro that metastatic melanoma cells and invasive uveal melanoma cells (unlike normal melanocytes or poorly differentiated melanomas) could generate this pseudovascular growth pattern, and proposed that melanoma cells might undergo genetic reversion to a pluripotent (embryonic-like) genotype. The authors also suggested that melanoma cells could generate pseudovascular changes that would facilitate tumor invasion independently of tumor angiogenesis.
The 4 cases of angiomatoid (pseudovascular) melanoma reported to date have all been characterized by aggressive behavior. The case described herein is the second report of primary cutaneous melanoma with an angiomatoid pattern. The first case was reported by Baron et al.3 in an 84-year-old man with a desmoplastic melanoma containing areas with an angiomatoid pattern. The melanoma was located in the periorbital region and had recurred several times. At the time of writing, our patient has shown no signs of metastasis.
Diagnosis of angiomatoid melanoma must be confirmed by immunohistochemistry and identification of neoplastic cells with melanocytic markers. The histologic presentation of this variant of melanoma poses numerous challenges and can lead to erroneous pathologic diagnosis as it can be confused with angiosarcoma or pseudovascular squamous cell carcinoma.7
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ramos-Rodríguez G, Ortiz-Hidalgo C. Melanoma primario cutáneo angiomatoide. Un patrón morfológico excepcional en los melanomas de piel. Presentación de caso con revisión de la literatura. Actas Dermosifiliogr. 2015;106:e13–e17.